Lipid Lowering Therapy in Primary Prevention: Where Are We?
Azizul Hoque
Division of Cardiology, Emory University School of Medicine, Atlanta, USA
Corresponding Authors: Azizul Hoque
Correspondence: Azizul Hoque, M.D, Ph.D. Division of Cardiology Emory University School of Medicine Emory Heart & Vascular Center 1400 Wellbrook Circle, Suite 103 Conyers, GA 30012, E-mail: azizul.hoque@emoryhealthcare.orgPurpose of Review: Apart from lifestyle modifications, lipid lowering strategies have become the cornerstone in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Although multiple primary prevention guidelines are available, a significant gap exists between evidence-based guidelines and day-to-day practice. Not only adherence to guideline-based treatment but achieving guideline-directed statin intensity are still suboptimal. Based on recent data, cardiovascular outcome benefits are still observed with profound lowering of low density lipoprotein cholesterol (LDL-C), to levels lower than current guideline goals. With better understanding of signaling molecules, genetics and development of advanced tools, lipid lowering strategies have focused more on biological, RNA-, and gene-related approaches.
Recent Findings: Over the last decade, lipid lowering strategies have shifted from targeting HMG-CoA reductase to focusing on the proprotein convertase subtilisin/kexin type 9 (PCSK9) protein. By binding with low density lipoprotein receptors (LDL-R) on hepatocytes, the PCSK9 protein blocks the recirculation of the LDL-R and plays an integral role in LDL-C clearance. Data from recent PCSK9 trials show that profound reduction of LDL-C could be achieved with PCSK9 monoclonal antibodies and there is no lower limit of LDL-C below which it does not show cardiovascular risk benefits. Currently, preliminary phase III trials are assessing the safety and efficacy of PCSK9 vaccines with purpose of inducing autoantibody production to neutralize the PCSK9 protein. Phase III trials of small interfering RNA, which interrupts the production of PCSK9 protein, are also ongoing. In addition, a recent PCSK9 gene editing study in primates has shown sustained reduction in LDL-C.
Summary: Since ASCVD is a life-long vascular remodeling process, early initiation and aggressive lowering of LDL-C is imperative to achieve sustained benefits in cardiovascular outcomes. Novel lipid lowering therapies targeting the PCSK9 protein, including its neutralization by monoclonal antibodies, halting its production, or targeting the PCSK9 gene itself, are promising lipid management strategies which could prevent and potentially eradicate ASCVD.
Keyword: primary prevention cardiovascular disease statin PCSK9 lipid lowering strategy.
Lipid Lowering Therapy in Primary Prevention: Where Are We?
Azizul Hoque
Division of Cardiology, Emory University School of Medicine, Atlanta, USA
Corresponding Authors: Azizul Hoque
Correspondence: Azizul Hoque, M.D, Ph.D. Division of Cardiology Emory University School of Medicine Emory Heart & Vascular Center 1400 Wellbrook Circle, Suite 103 Conyers, GA 30012, E-mail: azizul.hoque@emoryhealthcare.org
Introduction
Atherosclerotic cardiovascular disease (ASCVD) is a progressive life-long disease that may lead to cardiovascular (CV) events such as myocardial infarction (MI), ischemic stroke, or peripheral artery disease (PAD). Despite tremendous progress in research and treatment, ASCVD remains the leading cause of death not only in the United States but in different parts of the world.1,2 The World Health Organization estimates that 32% of mortality worldwide is due to ASCVD, and 85% of premature ASCVD is preventable with aggressive risk factor modification and lifestyle changes.3,4 Prior evidence clearly indicates that the successful reduction of ASCVD depends not only on lifestyle modification, but also to a greater degree on interventions to reduce the lifelong exposure to highly atherogenic lipid particles,5,6 which have become the targets of current and future lipid lowering strategies.
Substrates for Development of ASCVD
It has been well established that the low-density lipoprotein cholesterol (LDL-C),7 apolipoprotein B (Apo-B)8 and lipoprotein a [(Lp(a)]9 are the major atherogenic substrates for the development of ASCVD. As shown by Ference et al., large scale meta-analyses of Mendelian randomized studies, prospective cohort studies, and randomized controlled trials clearly established a direct relationship between LCL-C and ASCVD.7 Risk of CVD mortality and non-fatal MI can be reduced by 20-25% just by reducing the LDL-C by 1 mmol/L.5
ASCVD risk is reflected by the total number of atherogenic particles, not by the cholesterol content. One molecule of Apo-B is attached to each of the atherogenic lipid particles: LDL-C, Lp(a), intermediate density lipoprotein, and very low-density lipoprotein. Therefore, measuring the serum concentration of Apo-B provides a better estimate of the total atherogenicity of the lipid profile. As shown by Pencina et al. in the Framingham Offspring Study, Apo-B > 100 confers higher CVD risk independent of the LDL-C level.8
Erqou et al. in 2009 demonstrated that increased Lp(a) is associated with increased risk of MI, ischemic stroke, aortic stenosis, and mortality.9 ASCVD risk increases with Lp(a) level > 30 mg/dL, is substantially higher at levels > 50 mg/dL, and has the worst prognosis, similar to heterozygous familial hypercholesterolemia with Lp(a) level > 180 mg/dL.9 The absolute risk of major adverse cardiovascular events has been shown to have a proportional relationship to the concentration of Lp(a).10 Recent meta-analyses of prospective, population-based studies show that increased Lp(a) is associated with higher risk of ASCVD, and this relationship is independent of the level of other lipoproteins, including LDL-C.11 Unless specifically treated, Lp(a) concentration is not influenced by age, sex, lifestyle factors, and is regulated by a single gene locus LPA.11 Recently, Trinder et al. demonstrated that Lp(a) concentration does not change much over time, and a single measurement of Lp(a) molar concentration is a good low cost screening tool for CV risk assessment and can redefine risk in individuals with family history of premature CAD.12
Shortcomings of Current Prediction Modeling and Guideline Recommendations for Primary Prevention
To identify asymptomatic individuals at risk for future CV events, several predictive models have been developed, such as the Framingham Score Model,13 Pooled Cohort Equations (PCE),14,15 Systemic Coronary Risk Estimation 2 (SCORE2),16 and Systemic Coronary Risk Estimation 2-Older Persons (SCORE2-OP).17
The use of statins in primary prevention of ASCVD has been well established14,18,19 and is recommended for specific risk groups by major prevention guidelines internationally: 2019 American College of Cardiology/American Heart Association (ACC/AHA) guideline,15 2021 European Society of Cardiology (ESC) guideline,20 Canadian guideline,21 and Chinese guideline.22 The 2019 ACC/AHA guidelines on primary prevention recommend starting statin therapy in high- and specific intermediate-risk groups as shown in Table 1, and additional risk-enhancing factors were added to further redefine risks in borderline and intermediate risk groups15 and it is known that PCE-based risk scoring can both under-and over-estimate CVD risk.23-27. Despite being widely used, the current risk assessment models do not have precise diagnostic accuracy in predicting high-risk individuals who are going to have their first CV event.25,28 Coronary calcium score (CACS) has also become an important tool and is incorporated in the 2019 ACC/AHA primary prevention guidelines to redefine ASCVD risk to accurately identify those individuals at both high- and low-risk for a CV event.15
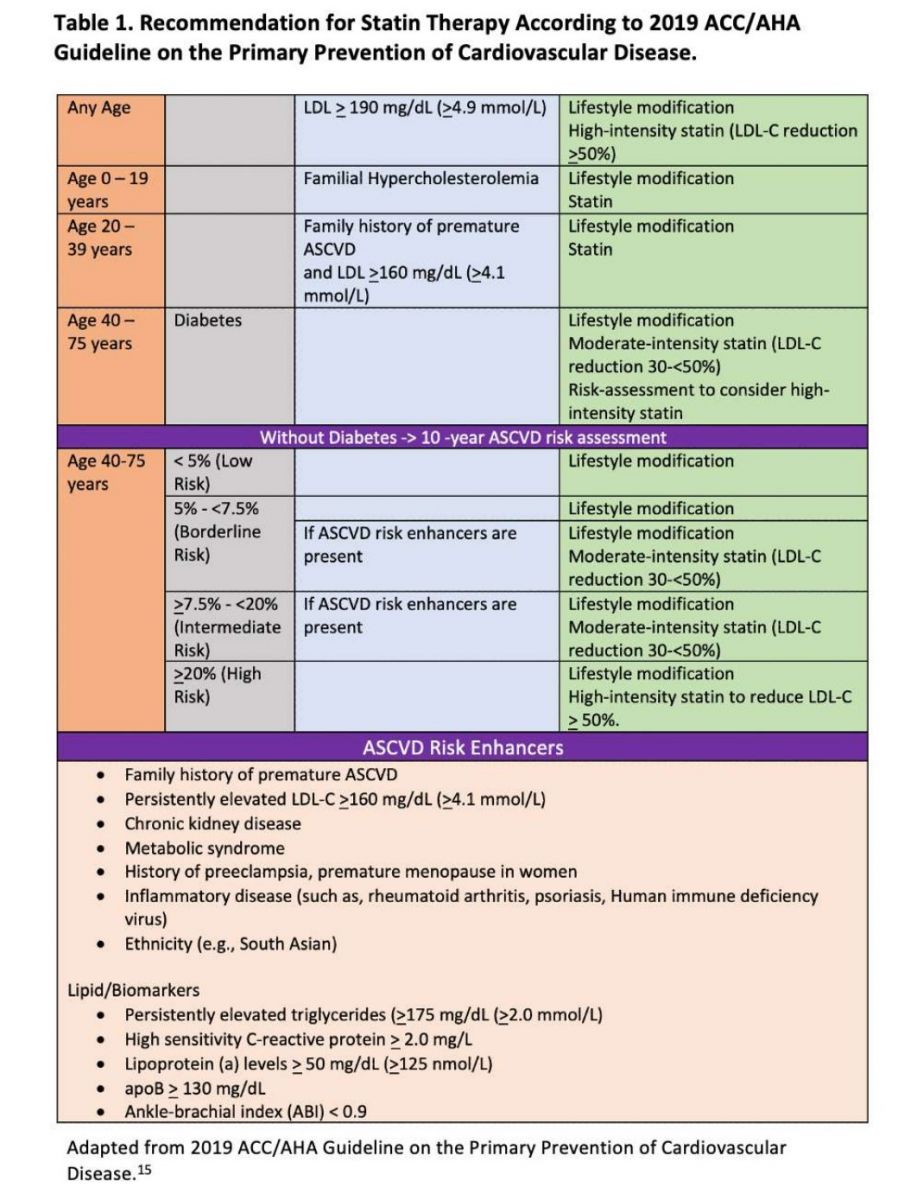
Role of Coronary Artery Calcium Score (CACS) in Redefining CV Risk
Recent review of multiple studies over the last 30 years by Drs. Khurram Nasir and Miguel Cainzos-Achirica has shown a clear relationship between elevated CACS and ASCVD events.29 CAC registry data also showed that patients with CACS > 0 AU benefitted with statin therapy with significant reduction in CV events compared to no statin.30 Data from a recent Australian study31 of 1000 asymptomatic individuals with strong family history of premature CAD showed that of the individuals initially classified at low risk, 77% had a CACS above 100 AU, and 19% of subjects initially thought to be at moderate risk had a CACS of 0. There is growing evidence that the quantification of CACS can redefine risk assessment to identify individuals who could benefit from intensity-appropriate statin therapy. On the other hand, a zero CACS can redefine an individual’s CV risk to the low end and can help reduce patient’s concerns, particularly in those who are reluctant or unable to tolerate a statin if otherwise indicated by initial risk assessment. As incorporated in newer guidelines, the quantification of CACS appeared to be the best predictor of ASCVD events in the intermediate risk group over a 10-year period.15 A recently published randomized controlled trial comparing a CACS-versus PCE risk score-based strategy also validated the concept that CACS-guided strategy is more cost-effective, could be individualized, and is helpful in motivating patients to start and adhere to long-term statin therapy.32
Utilization of CACS
Based on 2019 ACC/AHA primary prevention guidelines15 and the National Heart Foundation of Australia position statement,33 an approach to CAC scoring is depicted in Figure 1. First, a 10-year ASCVD risk is calculated using either the PCE15 or ESC SCORE216 risk calculator. CACS is not recommended for patients who are already defined as high absolute CV risk.34,35 In moderate-risk patients when initial risk status is close to high-risk, CACS is helpful to decide the intensity of the statin therapy. A CACS of 0 AU can redefine a moderate risk individual to low-risk status. However, it does not rule out non-calcified plaque. Patients with a history of smoking, diabetes, and family history of premature coronary artery disease may have non-calcified plaque, and the CV risk should not be underestimated even if CACS is zero.15,36,37 Patients with CACS 1-99 AU and 99 AU or >75th percentile , if risk enhancers are present, should qualify for moderate-intensity statin therapy. CACS of > 99 AU or >75th percentile categorizes the patient to high absolute risk. Repeat CACS is not necessary in this group, and patients should follow risk management strategies according to guidelines.

Coronary Computed Tomography Angiography and Detection of Early Atherosclerosis
The new generation of coronary computed tomography angiography (CCTA) scanners with higher resolution and low radiation exposures are gaining more attention since they can evaluate not only the extent of calcified and non-calcified plaque burden but also can characterize the composition of atherosclerotic plaques.38 Low attenuation plaque is associated with future CV risk and could be mitigated with statin therapy or other preventive measures.39 On the other hand, coronary artery calcification reflects more advanced atherosclerotic plaque. Though plaque burden itself might regress with aggressive lipid lowering therapy, calcification does not decrease but rather increases with statin therapy.40 When CACS is already above 99 AU, repeating CACS does not add any further incremental value. Recently, a strong association of subclinical atherosclerosis and gene score has been shown in a cohort study of young adults by Natarjan et al.41 In younger patients with strong family history of premature CAD or with familial hypercholesterolemia, CCTA may be helpful to identify patients for whom aggressive intervention with lipid-lowering therapy along with lifestyle modification should be initiated.
How Early the Treatment and How Low the LDL-C?
Different studies have shown that aggressive risk factor management and treatment of cholesterol can stabilize and to some degree, regress atherosclerotic plaque.42,43 The FOURIER trial44 showed that even LDL-C levels as low as 10 mg/dL showed CV benefits. The ODYSSEY-OUTCOMES trial45 also showed all-cause mortality benefit with LDL-C levels as low as 30 mg/dL. Data from these PCSK9 inhibitor trials clearly show that in high-risk groups, profound reduction in LDL-C levels substantially reduces the risk of CV events and there is no lower limit of LDLC below which CV benefit is not shown. However, some residual risk of acute CV events persists even when LDL levels are greatly reduced.44,46,47. Despite possible regression of atherosclerotic plaque with aggressive lipid lowering, the necrotic core still exists, reflecting the residual CV risk. It is well known that the atherosclerotic process starts early in adulthood as fatty streaks.48-50 In animal models, lowering LDL-C levels below 25 mg/dL could completely regress fatty streaks and normalize endothelial cell function.51 Based on the cumulative data, intervening on these early lesions is gaining attraction with the goal to achieve complete resolution of the atherosclerotic process.52-55 It is becoming clearer that in primary prevention, apart from healthy diet, exercise and lifestyle changes, the focus should be on slowing the progression of the atherosclerotic disease process as early as possible to prevent future CV events.56
With that hypothesis in mind, the Eliminate Coronary Artery Disease (ECAD) trial57 was launched in 2014 and included younger age groups (35-50 years for men and 45-59 years for women) with at least one risk factor, such as smoking, hypertension, obesity, family history of CAD, and South Asian ancestry, with follow-up over a 10-year period. The trial was designed to validate the concept that treating early and keeping the LDL-C low could not only abort the atherosclerotic plaque formation but also halt the progression of the existing disease. Similarly, the proposed CURE ATHERO trial58 by Robinson et al., included even younger age groups (25 - 55 years) with target LDL-C goal of 20 - 40 mg/dL and hypothesized that the clinical burden of atherosclerosis might be eliminated at its early stages by aggressively lowering the LDL-C. Recent data suggest that lower LDL-C, earlier treatment, and sustained reduction of the LDLC are important to achieve a meaningful CV risk benefit. Naturally, the following questions arise: what are the cost and side-effects profile with long-term use of lipid-lowering agents?
Statin Side Effects
A recent systematic review of 62 randomized controlled trials revealed that statins did not significantly increase the incidence of muscle disorder or diabetes, though they were associated with selfreported muscle symptoms.59 This analysis also showed that statin-induced adverse effects were not associated with the type of statin and dose, except for atorvastatin for which a dose-dependent effect on liver dysfunction was noted.59 In recent PCSK9 trials in which the achieved LDL-C levels were very low, no significant difference was noted in the treated versus control groups with regard to potential side effects,such as new-onset diabetes, hemorrhagic stroke, or new onset dementia.60-65 As reported by Cai et al., the incidence of clinically relevant muscle symptoms in statin treated patients was not significantly different compared to that of the control group, suggesting that reported muscle symptoms with statins may be, at least in part, attributable to patients’ perception or the socalled “nocebo” effect.59 The investigators on behalf of the Lipid and Blood Pressure Meta-Analysis Collaboration and the International Lipid Expert Panel recently published a large meta-analysis including 176 studies (112 randomized controlled trials and 64 cohort studies) worldwide which showed that the prevalence of statin intolerance was low (9.1%) and even lower (5.9%) when using European Society of Atherosclerosis criteria.66 Physicians should carefully evaluate patients’ symptoms to see whether they are truly related to statins or rather patients’ perception. Proactive, continuing physician-patient discussion is essential to ensure patients are not unnecessarily and preemptively taken off of treatment proven to reduce CV risk.
Statins in Elderly
Though benefits of statins in patients 75 years or older are well established for the secondary prevention of ASCVD events and CV mortality,67-70 the LipidLowering Trial component of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) showed no benefit of pravastatin in primary prevention in individuals aged 75 years or older.71 In a large cohort study from the Catalan Database in Spain, Ramos et al. showed that among participants above age 74 years without type 2 diabetes, statin treatment did not reduce ASCVD events or all-cause mortality.72 Patients with type 2 diabetes in this subgroup, however, had significant reduction in ASCVD events and all-cause mortality and this beneficial effect was markedly diminished with age above 85 years.72 Current ACC/AHA guidelines recommend assessment of risk status in patients 75 years or older and encourage physicianpatient discussion to decide whether to continue or initiate statin treatment.15
Gap between Guidelines and Lipid Control
A recent ESC EURObservational Research Program EUROASPIRE V survey in 16 European countries showed that in the primary care setting, at least 35% of patients with high ASCVD risk failed to achieve adequate lifestyle, blood pressure, lipid, and glycemic controls as defined by recent guidelines on primary prevention of CVD,73 A significant gap exists between evidenced-based guidelines and day-to-day clinical practice as demonstrated by multiple other surveys in the US, Europe, and other parts of the world.74-78 Statin therapy is highly cost-effective,79 and the number needed to treat is only 10 to prevent 1 ASCVD event.80 Despite the efficacy of statins and emphasis in guidelines, a recent large scale primary prevention study in the US by Saeed et al. showed that 30% of high-risk and 41% of intermediate-risk group individuals who required statin therapy according to guidelines never received the treatment over a 6 year follow up period and among those who did receive statin, it took up to 2 years to achieve the guidelinedirected statin intensity.81 Similar data from a recent Veterans Affairs primary prevention cohort study showed that about one-third of statin eligible patients did not receive any statin prescription over a 10-year follow up period.82 In practice, multiple issues with statin therapy are well-identified: 1) lack of effective provider-patient discussion; 2) suboptimal adherence to guideline-based treatment and also achieving guideline-directed statin intensity; 3) patient’s concerns about statin side effects and reluctance to continue long-term treatment; 4) lack of adequate follow up, particularly in low- and middle-income countries; and 5) patients’ lack of adherence to medication. As evidenced by data from multiple large cohort studies, physician-patient interaction should be more proactive to address these areas to achieve a sustained meaningful impact on CV risk reduction.
Novel Lipid Lowering Strategies
Over the last several decades, multiple randomized controlled trials have shown that statins reduce CV events.14,18,19,83 Meta-analysis of key statins by Nayak et al. also revealed the post-trial legacy effects of statins on CVD mortality and all-cause mortality in primary prevention.84 However, recent advancement of understanding of signaling molecules, genetics and analytical tools has shifted the focus of lipid-lowering targets to a proprotein convertase subtilisin/kexin type 9 (PCSK9) protein, which is primarily produced in hepatocytes.85-89 The PCSK9 protein binds to the low density lipoprotein receptors (LDL-R) on the surface of hepatocytes and blocks the recirculation of LDL-R by enhancing its lysosomal degradation.90 LDL-R on hepatocytes generally scavenge circulating LDL-C particles and transport them into hepatocytes. By inhibiting PCSK9, the destruction of LDL-R is interrupted,90 thereby allowing the presence of increased LDL-R on surface of hepatocytes. This in turn causes more LDL-C particles to be removed from circulation. Recent data on monoclonal antibodies, which bind to PCSK9 protein, have shown profound reduction in LDL-C resulting in significant CV outcome benefits in secondary prevention trials.44,47 The PCSK-9 antibodies are generally given twice a month subcutaneously but remain costly, limiting affordability and thus long-term adherence. Recently, small interfering RNA (si-RNA), such as inclisiran, which reduces the hepatic synthesis of PCSK9 protein, was studied in ORION 10 and ORION 11 phase III randomized controlled trials and showed up to 50% reduction in LDL-C.91
significant and sustained lowering of LDL-C in primates.93 Since the first randomized controlled trial of statins in 1984, lipid-lowering strategies have shifted to biological and RNA-directed approaches over the last few decades, and most recently, gene editing tools have joined the arsenal of lipid management with the aim of profound and sustained lowering of LDL-C (Figure 2). However, the efficacy, safety, and translational research data of these newest RNA- and gene- directed lipid lowering therapies in humans are still pending
Conclusions
Since ASCVD is a life-long progressive disease, a clear consensus is now emerging to initiate lipid lowering treatment earlier in life to prevent development of ASCVD and halt disease progression. Currently, there is a significant gap between guideline recommendations and achieving the target LDL-C lowering goal in patients. Along with lifestyle modifications, healthy diet and exercise, primary
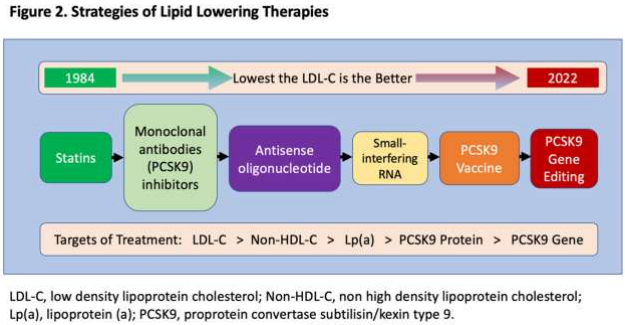
The advantage of si-RNA is twice a year administration and lower cost compared to PCSK9 monoclonal antibodies. In addition, several preclinical studies have focused on PCSK9 vaccines which will induce production of autoantibodies against the PCSK9 protein, thereby decreasing the degradation of LDL-R. 92 Recently, gene editing technology, such as Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) editing tool is being used to target the PCSK9 gene. Musunuru et al. demonstrated that CRISPR base editing of PCSK9 gene had caused a prevention is focusing on LDL-C management with the following principle: “the earlier, lower, and longer, the better.” It is well-known that current risk scoring models can both under- and over-estimate CV risk. New generation imaging modalities, such as high-resolution, low-radiation CCTA, are becoming more adept at identifying high-risk individuals. Primary prevention should focus not only on high-risk individuals but also be broadened to include low and intermediate-risk groups which comprise the majority of the population to achieve a large scale ASCVD mortality and morbidity benefit. Most importantly, management of CV risk factor should be individualized and patient-centered with an emphasis on physician-patient communication. Future lipidlowering therapies including PCSK9 vaccines and gene editing therapies are highly promising. However, their safety, efficacy, and outcome benefits in humans have yet to be proven.
Key Points
- ASCVD is a life-long vascular remodeling process.
- Earlier initiation of lipid lowering treatment, lower LDL-C, and longer the treatment is associated with improved CV outcomes.
- Physician-patient communication should be proactive to decrease the gap between guidelinedirected recommendations and day-to-day practice.
- Lipid-lowering treatment strategies have shifted to targeting the PCSK9 protein, which plays an integral role in the availability of LDL-R which remove LDL-C from circulation.
- Production of native PCSK9 antibody by a PCSK9 vaccine, interruption of PCSK9 production by small interfering RNA or PCSK9 gene editing technology are promising future therapies which could halt and potentially eradicate ASCVD. However, their safety, efficacy, and outcome benefits in humans are yet to be proven.
Declarations
Conflict of Interes
Dr. Azizul Hoque has nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
1. Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 03 03 2020;141(9):e139-e596. doi:10.1161/CIR.0000000000000757
2. Dagenais GR, Leong DP, Rangarajan S, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 03 07 2020;395(10226):785-794. doi:10.1016/S0140- 6736(19)32007-0
3. World Heath Organization (WHO). Cardiovascular diseases(CVDs): Key facts. Updated June 11, 2021. Accessed April 18, 2022, https://www.who.int/newsroom/fact-sheets/detail/cardiovascular-diseases-(cvds)
4. Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155?722 individuals from 21 high-income, middle-income, and lowincome countries (PURE): a prospective cohort study. Lancet. 03 07 2020;395(10226):795-808. doi:10.1016/S0140-6736(19)32008-2
5. Penson PE, Pirro M, Banach M. LDL-C: lower is better for longer-even at low risk. BMC Med. 10 08 2020;18(1):320. doi:10.1186/s12916-020-01792-7
6. Gidding SS, Allen NB. Cholesterol and Atherosclerotic Cardiovascular Disease: A Lifelong Problem. J Am Heart Assoc. 06 04 2019;8(11):e012924. doi:10.1161/JAHA.119.012924
7. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. Aug 21 2017;38(32):2459-2472. doi:10.1093/eurheartj/ehx144
8. Pencina MJ, D'Agostino RB, Zdrojewski T, et al. Apolipoprotein B improves risk assessment of future coronary heart disease in the Framingham Heart Study beyond LDL-C and non-HDL-C. Eur J Prev Cardiol. Oct 2015;22(10):1321-7. doi:10.1177/2047487315569411
9. Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. Jul 22 2009;302(4):412- 23. doi:10.1001/jama.2009.1063
10. Madsen CM, Kamstrup PR, Langsted A, Varbo A, Nordestgaard BG. Lipoprotein(a)-Lowering by 50 mg/dL (105 nmol/L) May Be Needed to Reduce Cardiovascular Disease 20% in Secondary Prevention: A Population-Based Study. Arterioscler Thromb Vasc Biol. 01 2020;40(1):255- 266. doi:10.1161/ATVBAHA.119.312951
11. Melita H, Manolis AA, Manolis TA, Manolis AS. Lipoprotein(a) and Cardiovascular Disease: A Missing Link for Premature Atherosclerotic Heart Disease and/or Residual Risk. J Cardiovasc Pharmacol. 01 01 2022;79(1):e18-e35. doi:10.1097/FJC.0000000000001160
12. Trinder M, Paruchuri K, Haidermota S, et al. Repeat Measures of Lipoprotein(a) Molar Concentration and Cardiovascular Risk. J Am Coll Cardiol. 02 22 2022;79(7):617-628. doi:10.1016/j.jacc.2021.11.055
13. D'Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. Feb 12 2008;117(6):743-53. doi:10.1161/CIRCULATIONAHA.107.699579
14. Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. Jun 24 2014;129(25 Suppl 2):S49-73. doi:10.1161/01.cir.0000437741.48606.98
15. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 09 10 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
16. collaboration SwgaECr. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J. 07 01 2021;42(25):2439-2454. doi:10.1093/eurheartj/ehab309
17. collaboration S-OwgaECr. SCORE2-OP risk prediction algorithms: estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur Heart J. 07 01 2021;42(25):2455-2467. doi:10.1093/eurheartj/ehab312
18. Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. Aug 05 2014;64(5):485-94. doi:10.1016/j.jacc.2014.02.615
19. Khera AV, Everett BM, Caulfield MP, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation. Feb 11 2014;129(6):635-42. doi:10.1161/CIRCULATIONAHA.113.004406
20. Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 09 07 2021;42(34):3227-3337. doi:10.1093/eurheartj/ehab484
21. Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can J Cardiol. 08 2021;37(8):1129-1150. doi:10.1016/j.cjca.2021.03.016
22. Chinese Society of Cardiology of Chinese Medical Association CDPaRCoCAo. Chinese Guideline on the Primary Prevention of Cardiovascular Diseases. Cardiology Discovery. 2021;1(2):70-104. doi:10.1097/cd9.0000000000000025
23. Dalton JE, Perzynski AT, Zidar DA, et al. Accuracy of Cardiovascular Risk Prediction Varies by Neighborhood Socioeconomic Position: A Retrospective Cohort Study. Ann Intern Med. Oct 03 2017;167(7):456-464. doi:10.7326/M16-2543
24. Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. Apr 09 2014;311(14):1406-15. doi:10.1001/jama.2014.2630
25. DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. Feb 17 2015;162(4):266-75. doi:10.7326/M14-1281
26. Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the Atherosclerotic Cardiovascular Risk Equation in a Large Contemporary, Multiethnic Population. J Am Coll Cardiol. 05 10 2016;67(18):2118-2130. doi:10.1016/j.jacc.2016.02.055
27. Yadlowsky S, Hayward RA, Sussman JB, McClelland RL, Min YI, Basu S. Clinical Implications of Revised Pooled Cohort Equations for Estimating Atherosclerotic Cardiovascular Disease Risk. Ann Intern Med. 07 03 2018;169(1):20-29. doi:10.7326/M17-3011
28. Zeitouni M, Nanna MG, Sun JL, Chiswell K, Peterson ED, Navar AM. Performance of Guideline Recommendations for Prevention of Myocardial Infarction in Young Adults. J Am Coll Cardiol. 08 11 2020;76(6):653- 664. doi:10.1016/j.jacc.2020.06.030
29. Nasir K, Cainzos-Achirica M. Role of coronary artery calcium score in the primary prevention of cardiovascular disease. BMJ. 05 04 2021;373:n776. doi:10.1136/bmj.n776
30. Mitchell JD, Fergestrom N, Gage BF, et al. Impact of Statins on Cardiovascular Outcomes Following Coronary Artery Calcium Scoring. J Am Coll Cardiol. 12 25 2018;72(25):3233-3242. doi:10.1016/j.jacc.2018.09.051
31. Venkataraman P, Stanton T, Liew D, et al. Coronary artery calcium scoring in cardiovascular risk assessment of people with family histories of early onset coronary artery disease. Med J Aust. 08 2020;213(4):170-177. doi:10.5694/mja2.50702
32. Muhlestein JB, Knowlton KU, Le VT, et al. Coronary Artery Calcium Versus Pooled Cohort Equations Score for Primary Prevention Guidance: Randomized Feasibility Trial. JACC Cardiovasc Imaging. Dec 09 2021;doi:10.1016/j.jcmg.2021.11.006
33. Jennings GL, Audehm R, Bishop W, et al. National Heart Foundation of Australia: position statement on coronary artery calcium scoring for the primary prevention of cardiovascular disease in Australia. Med J Aust. May 2021;214(9):434-439. doi:10.5694/mja2.51039
34. Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. Oct 13 2015;66(15):1657-68. doi:10.1016/j.jacc.2015.07.066
35. Okwuosa TM, Greenland P, Ning H, et al. Distribution of coronary artery calcium scores by Framingham 10-year risk strata in the MESA (Multi-Ethnic Study of Atherosclerosis) potential implications for coronary risk assessment. J Am Coll Cardiol. May 03 2011;57(18):1838- 45. doi:http://10.1016/j.jacc.2015.07.06610.1016/j.jacc.2010.11.053
36. Lloyd-Jones DM, Braun LT, Ndumele CE, et al. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. Circulation. 06 18 2019;139(25):e1162-e1177. doi:10.1161/CIR.0000000000000638
37. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/AP hA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 06 18 2019;139(25):e1082-e1143. doi:10.1161/CIR.0000000000000625
38. Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014 Sep-Oct 2014;8(5):342- 58. doi:10.1016/j.jcct.2014.07.003
39. Nadjiri J, Hausleiter J, Jähnichen C, et al. Incremental prognostic value of quantitative plaque assessment in coronary CT angiography during 5 years of follow up. J Cardiovasc Comput Tomogr. 2016 Mar-Apr 2016;10(2):97- 104. doi:10.1016/j.jcct.2016.01.007
40. Nakahara T, Dweck MR, Narula N, Pisapia D, Narula J, Strauss HW. Coronary Artery Calcification: From Mechanism to Molecular Imaging. JACC Cardiovasc Imaging. 05 2017;10(5):582-593. doi:10.1016/j.jcmg.2017.03.005
41. Natarajan P, Young R, Stitziel NO, et al. Polygenic Risk Score Identifies Subgroup With Higher Burden of Atherosclerosis and Greater Relative Benefit From Statin Therapy in the Primary Prevention Setting. Circulation. May 30 2017;135(22):2091-2101. doi:10.1161/CIRCULATIONAHA.116.024436
42. Nicholls SJ, Puri R, Anderson T, et al. Effect of Evolocumab on Progression of Coronary Disease in StatinTreated Patients: The GLAGOV Randomized Clinical Trial. JAMA. 12 13 2016;316(22):2373-2384. doi:10.1001/jama.2016.16951
43. Stegman B, Shao M, Nicholls SJ, et al. Coronary atheroma progression rates in men and women following high-intensity statin therapy: A pooled analysis of REVERSAL, ASTEROID and SATURN. Atherosclerosis. 11 2016;254:78-84. doi:10.1016/j.atherosclerosis.2016.09.059
44. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 05 04 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
45. Steg PG, Szarek M, Bhatt DL, et al. Effect of Alirocumab on Mortality After Acute Coronary Syndromes. Circulation. 07 09 2019;140(2):103-112. doi:10.1161/CIRCULATIONAHA.118.038840
46. Sabatine MS, De Ferrari GM, Giugliano RP, et al. Clinical Benefit of Evolocumab by Severity and Extent of Coronary Artery Disease: Analysis From FOURIER. Circulation. 08 21 2018;138(8):756-766. doi:10.1161/CIRCULATIONAHA.118.034309
47. Jukema JW, Szarek M, Zijlstra LE, et al. Alirocumab in Patients With Polyvascular Disease and Recent Acute Coronary Syndrome: ODYSSEY OUTCOMES Trial. J Am Coll Cardiol. 09 03 2019;74(9):1167-1176. doi:10.1016/j.jacc.2019.03.013
48. Wissler RW, Strong JP. Risk factors and progression of atherosclerosis in youth. PDAY Research Group. Pathological Determinants of Atherosclerosis in Youth. Am J Pathol. Oct 1998;153(4):1023-33. doi:10.1016/s0002- 9440(10)65647-7
49. McGill HC, Jr., Herderick EE, McMahan CA, et al. Atherosclerosis in youth. Minerva Pediatr. Oct 2002;54(5):437-47.
50. Fisher EA. Regression of Atherosclerosis: The Journey From the Liver to the Plaque and Back. Arterioscler Thromb Vasc Biol. Feb 2016;36(2):226-35. doi:10.1161/ATVBAHA.115.301926
51. Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. Feb 2008;5(2):91-102. doi:10.1038/ncpcardio1086
52. Packard CJ. Strategies to alter the trajectory of atherosclerotic cardiovascular disease. Curr Opin Lipidol. 12 2019;30(6):438-445. doi:10.1097/MOL.0000000000000643
53. Robinson JG, Ray K. Moving Toward the Next Paradigm for Cardiovascular Prevention. Circulation. Apr 19 2016;133(16):1533-6. doi:10.1161/CIRCULATIONAHA.116.022134
54. Graham I, Shear C, De Graeff P, et al. New strategies for the development of lipid-lowering therapies to reduce cardiovascular risk. Eur Heart J Cardiovasc Pharmacother. 04 01 2018;4(2):119-127. doi:10.1093/ehjcvp/pvx031
55. Robinson JG, Davidson MH. Can We Cure Atherosclerosis? Rev Cardiovasc Med. 2018;19(S1):S20- S24. doi:10.3909/ricm19S1S0003
56. Ference BA, Graham I, Tokgozoglu L, Catapano AL. Impact of Lipids on Cardiovascular Health: JACC Health Promotion Series. J Am Coll Cardiol. Sep 4 2018;72(10):1141-1156. doi:10.1016/j.jacc.2018.06.046
57. Domanski MJ, Fuster V, Diaz-Mitoma F, et al. Next Steps in Primary Prevention of Coronary Heart Disease: Rationale for and Design of the ECAD Trial. J Am Coll Cardiol. Oct 20 2015;66(16):1828-1836. doi:10.1016/j.jacc.2015.08.857
58. Robinson JG, Williams KJ, Gidding S, et al. Eradicating the Burden of Atherosclerotic Cardiovascular Disease by Lowering Apolipoprotein B Lipoproteins Earlier in Life. J Am Heart Assoc. 10 16 2018;7(20):e009778. doi:10.1161/JAHA.118.009778
59. Cai T, Abel L, Langford O, et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses. BMJ. 07 14 2021;374:n1537. doi:10.1136/bmj.n1537
60. Sabatine MS, Wiviott SD, Im K, Murphy SA, Giugliano RP. Efficacy and Safety of Further Lowering of LowDensity Lipoprotein Cholesterol in Patients Starting With Very Low Levels: A Meta-analysis. JAMA Cardiol. 09 01 2018;3(9):823-828. doi:10.1001/jamacardio.2018.2258
61. Gencer B, Marston NA, Im K, et al. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet. 11 21 2020;396(10263):1637-1643. doi:10.1016/S0140-6736(20)32332-1
62. Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. Oct 28 2017;390(10106):1962-1971. doi:10.1016/S0140-6736(17)32290-0
63. Giugliano RP, Keech A, Murphy SA, et al. Clinical Efficacy and Safety of Evolocumab in High-Risk Patients Receiving a Statin: Secondary Analysis of Patients With Low LDL Cholesterol Levels and in Those Already Receiving a Maximal-Potency Statin in a Randomized Clinical Trial. JAMA Cardiol. 12 01 2017;2(12):1385-1391. doi:10.1001/jamacardio.2017.3944
64. Guedeney P, Giustino G, Sorrentino S, et al. Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. Jul 03 2019;doi:10.1093/eurheartj/ehz430
65. Gencer B, Mach F, Guo J, et al. Cognition After Lowering LDL-Cholesterol With Evolocumab. J Am Coll Cardiol. 05 12 2020;75(18):2283-2293. doi:10.1016/j.jacc.2020.03.039
66. Bytyçi I, Penson PE, Mikhailidis DP, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J. Feb 16 2022;doi:10.1093/eurheartj/ehac015
67. Miettinen TA, Pyörälä K, Olsson AG, et al. Cholesterollowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Survival Study (4S). Circulation. Dec 16 1997;96(12):4211-8. doi:10.1161/01.cir.96.12.4211
68. Lewis SJ, Moye LA, Sacks FM, et al. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) trial. Ann Intern Med. Nov 01 1998;129(9):681-9. doi:10.7326/0003-4819-129-9-199811010-00002
69. Hunt D, Young P, Simes J, et al. Benefits of pravastatin on cardiovascular events and mortality in older patients with coronary heart disease are equal to or exceed those seen in younger patients: Results from the LIPID trial. Ann Intern Med. May 15 2001;134(10):931-40. doi:10.7326/0003- 4819-134-10-200105150-00007
70. Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ. Statins for secondary prevention in elderly patients: a hierarchical bayesian meta-analysis. J Am Coll Cardiol. Jan 01 2008;51(1):37-45. doi:10.1016/j.jacc.2007.06.063
71. Han BH, Sutin D, Williamson JD, et al. Effect of Statin Treatment vs Usual Care on Primary Cardiovascular Prevention Among Older Adults: The ALLHAT-LLT Randomized Clinical Trial. JAMA Intern Med. 07 01 2017;177(7):955-965. doi:10.1001/jamainternmed.2017.1442
72. Ramos R, Comas-Cufí M, Martí-Lluch R, et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ. 09 05 2018;362:k3359. doi:10.1136/bmj.k3359
73. Kotseva K, De Backer G, De Bacquer D, et al. Primary prevention efforts are poorly developed in people at high cardiovascular risk: A report from the European Society of Cardiology EURObservational Research Programme EUROASPIRE V survey in 16 European countries. Eur J Prev Cardiol. 05 08 2021;28(4):370-379. doi:10.1177/2047487320908698
74. Banegas JR, López-García E, Dallongeville J, et al. Achievement of treatment goals for primary prevention of cardiovascular disease in clinical practice across Europe: the EURIKA study. Eur Heart J. Sep 2011;32(17):2143-52. doi:10.1093/eurheartj/ehr080
75. Ludt S, Wensing M, Campbell SM, et al. The challenge of cardiovascular prevention in primary care: implications of a European observational study in 8928 patients at different risk levels. Eur J Prev Cardiol. Feb 2014;21(2):203-13. doi:10.1177/2047487312462798
76. lom DJ, Santos RD, Daclin V, et al. The challenge of multiple cardiovascular risk factor control outside Western Europe: Findings from the International ChoLesterol management Practice Study. Eur J Prev Cardiol. 09 2020;27(13):1403-1411. doi:10.1177/2047487319871735
77. Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation. Oct 23 2012;126(17):2105-14. doi:10.1161/CIRCULATIONAHA.112.096156
78. Wong ND, Patao C, Wong K, Malik S, Franklin SS, Iloeje U. Trends in control of cardiovascular risk factors among US adults with type 2 diabetes from 1999 to 2010: comparison by prevalent cardiovascular disease status. Diab Vasc Dis Res. Nov 2013;10(6):505-13. doi:10.1177/1479164113496828
79. Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-Year Risk Thresholds for Initiation of Statin Therapy for Primary Prevention of Cardiovascular Disease. JAMA. Jul 14 2015;314(2):142-50. doi:10.1001/jama.2015.6822
80. Mortensen MB, Nordestgaard BG. Statin Use in Primary Prevention of Atherosclerotic Cardiovascular Disease According to 5 Major Guidelines for Sensitivity, Specificity, and Number Needed to Treat. JAMA Cardiol. 11 01 2019;4(11):1131-1138. doi:10.1001/jamacardio.2019.3665
81. Saeed A, Zhu J, Thoma F, et al. Cardiovascular Disease Risk-Based Statin Utilization and Associated Outcomes in a Primary Prevention Cohort: Insights From a Large Health Care Network. Circ Cardiovasc Qual Outcomes. 09 2021;14(9):e007485. doi:10.1161/CIRCOUTCOMES.120.007485
82. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 11 19 2016;388(10059):2532-2561. doi:10.1016/S0140-6736(16)31357-5
83. Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. Nov 13 2010;376(9753):1670-81. doi:10.1016/s0140-6736(10)61350-5
84. Nayak A, Hayen A, Zhu L, et al. Legacy effects of statins on cardiovascular and all-cause mortality: a meta-analysis. BMJ Open. 10 04 2018;8(9):e020584. doi:10.1136/bmjopen-2017-020584
85. Abifadel M, Varret M, Rabès JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. Jun 2003;34(2):154-6. doi:10.1038/ng1161
86. Cohen JC, Boerwinkle E, Mosley TH, Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. Mar 23 2006;354(12):1264-72. doi:10.1056/NEJMoa054013
87. Kathiresan S. A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N Engl J Med. May 22 2008;358(21):2299-300. doi:10.1056/NEJMc0707445
88. Benjannet S, Hamelin J, Chrétien M, Seidah NG. Lossand gain-of-function PCSK9 variants: cleavage specificity, dominant negative effects, and low density lipoprotein receptor (LDLR) degradation. J Biol Chem. Sep 28 2012;287(40):33745-55. doi:10.1074/jbc.M112.399725
89. Tokgözo?lu L, Libby P. The dawn of a new era of targeted lipid-lowering therapies. European Heart Journal. 2022;doi:10.1093/eurheartj/ehab841
90. Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res. Dec 2012;53(12):2515-24. doi:10.1194/jlr.R026658
91. Ray KK, Wright RS, Kallend D, et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N Engl J Med. 04 16 2020;382(16):1507-1519. doi:10.1056/NEJMoa1912387
92. Study Assessing Safety, Immunogenicity and LDLc - Lowering Activity of 2 PCSK9 Targeting AFFITOPE Vaccines in Healthy Subjects (AFF012). ClinicalTrials.gov Identifier: NCT02508896. Accessed April 18, 2022, https://clinicaltrials.gov/ct2/show/NCT02508896
93. Musunuru K, Chadwick AC, Mizoguchi T, et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 05 2021;593(7859):429-434. doi:10.1038/s41586-021- 03534-y