Non-alcoholic fatty liver disease (NAFLD)
Ashraf Malek
Transplant Hepatologist
Medical Director, Liver Disease Center, Virtua Center for Liver Disease and Organ Transplantation, NJ. USA
Email: docpolash@gmail.com
Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the leading causes of chronic liver disease in adults (1) as the rate of obesity is increasing; the global prevalence of NAFLD has increased dramatically. It is now affecting one-fourth of the general population in the United States and rest of the world.
It has been projected, within next 20 years; NAFLD will become major cause of morbidity and mortality as well as leading indication of liver transplantation (3) . NAFLD not only place a strain on the medical system and its resources, it is also associated with 34% - 69% chance of dying over next 15 years compared to general population (4)
Definition
Generally, in healthy individual, hepatocytes may contain <5% fat. NAFLD is defined as macrovesicular fat accumulation in more than 5% hepatocytes in the absence of significant alcohol intake, no other etiology of liver disease, no secondary cause of fatty liver such as medications, HIV etc. Fat accumulation begins in zone 3 of hepatocytes, but may occupy entire acinus. NAFLD is a disease spectrum ranging from steatosis, steatohepatitis, and fibrosis and, eventually leading to cirrhosis.
NAFLD is divided into 2 histologic subtypes. (A) Nonalcoholic fatty liver (NAFL), which includes patients with isolated hepatic steatosis with or without mild non-specific inflammation (Fig-1). (B) Non-alcoholic steatohepatitis (NASH), which is characterized by presence of features of hepatocellular injury, such as hepatocyte ballooning degeneration, mild lobular inflammation with mixed inflammatory infiltrate, Mallory-denk bodies, megamitochondria (Fig – 2) with or without hepatic fibrosis (Fig – 3)
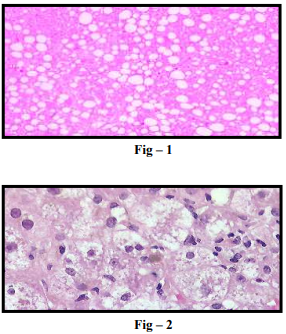
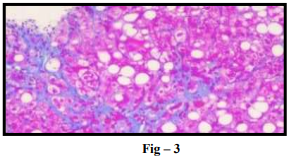
Predisposing Factors
NAFLD is considered to be hepatic manifestation of metabolic syndrome. Important risk factors for NAFLD include obesity, particularly central abdominal obesity (waist circumference >102 cm in men, >88 cm in women) phenotype, diabetes mellitus, hypertriglyceridemia, hypertension, and insulin resistance.
An ethnic variation in the distribution of NAFLD has been suggested. Hispanics have the highest prevalence of NAFLD (45%-58%), followed by whites (33%-44%) and blacks (24%-35%)(5). although the reason for ethnic variation is unknown, both lifestyle and genetic predisposition may play a role. Several studies have demonstrated that NAFLD and NASH are more common in men. (6) Women tend to develop NAFLD later in life. NAFLD has also become more common in pediatric population because of increase prevalence of Body Mass Index (BMI) in United States. An autopsy series of 742 children and adolescents 2-19 years showed overall NAFLD prevalence of 13% (7)
Pathogenesis Pathophysiology of NAFLD is very complex. Accumulation of lipid in the hepatocytes causes fatty infiltration. There are various pathways that can lead to hepatic steatosis.
These pathways include free fatty acid supply to hepatocytes, either from increase intake of fat in the diet or increase lipolysis from adipose tissue as well as increase denovo lipogenesis, decreased free fatty acid oxidation and decreased low density lipoprotein secretion in the liver. Triglycerides are the major type of lipids stored in the liver (15)
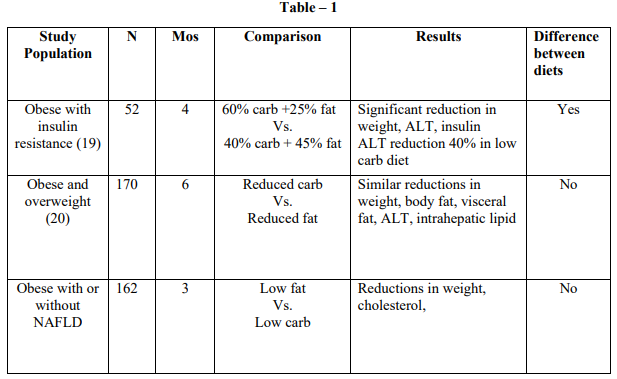
Once steatosis has developed, the liver is ‘sensitized’, and so an inflammatory response may be precipitated by a various stimuli. Two mechanism thought to be pivotal role in NASH pathogenesis (FIG- 4).
First, oxidative stress, and lipid peroxidation can result in cell death. Secondly, there is pro-inflammatory and cytokine mediated cell injury. Cell injury and cell death result in fibrogenesis and collagen turnover leading to hepatic fibrosis eventually leading to cirrhosis.
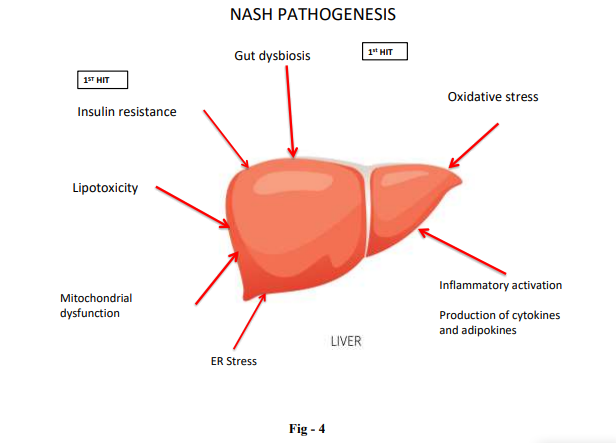
Clinical Feature and Diagnosis
Most patients with NAFLD and NASH are asymptomatic. Although vague right upper quadrant pain or discomfort, fatigue and malaise have been reported. Most common presentation is either elevation of ALT or elevation of both aspartate aminotransferase (AST) and ALT. Gammaglutamyltransferase level may be elevated in patients with NAFLD and has been associated with advance fibrosis (10) . All patients with metabolic risk factors should be screened for NAFLD with abdominal ultrasound.
In patients with NAFLD, ALT is neither indicative nor predictive of non-alcoholic NASH or fibrosis stage (8). Alanine Aminotransferase (ALT) can be normal in >50% individuals with NASH and 80% individual with NAFL (6). Conversely, ALT can be elevated in >50% individuals with NAFLD but without NASH (9)
Natural History and Prognosis
The natural history of NAFLD depends on histopathology at baseline (Fig – 5) NAFLD is a spectrum of clinicopathologic conditions with differential progression for the subtypes of NAFLD. Although overall small in number, numerous longitudinal studies have suggested that the vast majority of patients identified as having IFL (Isolated Fatty Liver) or NAFL on initial liver biopsy are at extremely low risk for the development of advance fibrosis (12) .
Data suggest that there does not appear to be an increased risk of death among patients with IFL compared with age and gender matched control (13) .
Once NASH is diagnosed on liver biopsy, data suggest that there is an increased risk of death compared with general population (13). Published longitudinal study on average progression of disease demonstrates that average progression rate of 11% during 15 year period (14) .
Retrospective study on 173 NAFLD patients, who had median follow up for 18.5 years, shows that liver related mortality of NASH cohort increased to 17.5% in comparison with only 2.7% in the non-NASHNAFLD cohort (11)

Treatment
Lifestyle modification with diet and exercise is the cornerstone of treatment of NASH and NAFL. Nutritional changes include calorie restriction and weight loss as well as macronutrient modification. The ideal pharmacotherapy for NASH has not been established yet.
Dietary and physical activity modification with gradual weight loss
Dietary modification to achieve moderate weight loss must not have quantitative but also have qualitative characteristics (16). The most current therapeutic trial lacks sufficient power to show clinical benefit. Therefore, composition of diet with modulation of macro and micronutrients is critical. According to international guidelines, the first step in treating NAFLD is to limit intake of calories of saturated fats, trans fatty acids and fructose. Conversely to increase intake of lean protein, fibers and poly-unsaturated fatty acids (17) .
Mediterranean diet (MD) is a useful dietary option to induce weight loss along with concurrent metabolic benefit for NAFLD. MD consists of low content of saturated fat and cholesterol and high content of polyunsaturated fatty acid, carbohydrates and fibers. Several cross-sectional and longitudinal studies have demonstrated beneficial effect of MD on NAFLD.
In an observational study Gelli et al (18) evaluated the efficacy of MD. 46 adults received clinical and dietary intervention with MD along with monthly nutritional counseling. Steatosis grade equal or higher than 2 were observed more than 80% patients and steatosis regressed in 20% patients
Few observational studies compare the efficacy between low carbohydrate and low fat diet (Table-1). Although initial promise, results are inconsistent. Regardless of diet pattern, weight loss is the key
Most clinical trials evaluated lifestyle interventions combine both diet and exercise recommendation. Villar-Gomez et al (32) conducted a prospective study of 293 patients with histologically proven NASH who were recommended lifestyle changes to reduce their weight over 52 weeks. Among 293 patients who underwent lifestyle changes for 52 weeks, 25% achieved resolution of NASH, 19% had regression of fibrosis. Importantly, patients who lost more than 10% of their weight, 90% had resolution of NASH and 45% had regression of fibrosis.
Coffee
There is inverse correlation between coffee consumption and decrease risk of liver disease. A meta-analysis was conducted of 16 studies involving more than 3000 coffee consumers compared with more than 13,000 non-consumers (21). The pooled result indicated that coffee consumption can significantly reduce the risk of hepatic fibrosis and cirrhosis. Coffee also reduces the risk of Hepatocellular carcinoma (HCC). Systemic review and meta-analysis was conducted involving 18 cohorts more than 2 million participants and 8 case-control studies involving 1825 cases and 4625 controls (22). The study found an extra 2 cups of coffee 35% reduction in the risk of HCC.
Bariatric Surgery
Lifestyle interventions, often not only difficult to achieve but also difficult to sustain. Surgical weight loss has been investigated in treatment of patients with NASH. RYGB (Roux-en-Y gastric bypass) is a malabsorptive type of bariatric surgery, investigated in multiple uncontrolled trials and have shown improvement of NASH (23, 24). A meta-analysis of 15 different studies have demonstrated 69.5% complete resolution of NASH, 81.3% improvement of steatohepatitis, and 65% improvement of fibrosis (25) .
However, patient with underlying cirrhosis due to NASH, especially with associated portal hypertension, bariatric surgery should be avoided because of potential risk of complications.
Pharmacotherapy
Currently, no medications have been approved by US FDA (Food and Drug Administration) or by the European medicine agency. Table 2 summarizes the lists of drugs should only be considered as an off-level indication and decision should be discussed explicitly with the patient, after carefully discussion of risks and benefits.
.png)
Conclusion
NAFLD is very common chronic liver disease with increasing prevalence around the world. Although, majority of patients with NAFLD have benign prognosis, patients with NASH have almost 10 times higher risk of advance fibrosis. Lifestyle interventions with dietary modification and regular exercise with goal of gradual weight loss is the cornerstone of treatment of NAFLD, although it is not sustainable in majority of the cases. With regards to pharmacological treatment, various medications can be used as an offlevel indication in carefully selecting patients after discussing potential risks and benefits. As the pathogenesis of NASH has been shown be complex and multifactorial, there are large numbers of treatments in phase 2/3 clinical trial in pipeline including agents directed against various pathogenic pathways such as steatosis and lipogenesis, immune modulation, oxidative stress, apoptosis and fibrosis.
1. Sayiner M, Koeing A, Henry L, et al. Epidemiology of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the United States and rest of the world. Clin. Liver Disease. 2016
2. Younossi ZM, Koeing AB, Sayiner M et al. Epidemiology and natural history of no-alcoholic fatty liver disease- Meta analytic assessment of prevalence incidence and out-comes. Hepatology 2016
3. Kenwar P, Kowdley KV. The metabolic syndrome and its influence on Nonalcoholic steatohepatitis. Clin Liver Dis. 2016:20:225-243
4. Patel V, Sanyal AJ, Sterling R. Clinical presentation and clinical evaluation in non-alcoholic fatty liver disease. Clin Liver Dis. 2016:20:277-292
5. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and non-alcoholic steatohepatitis among largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011; 140:124-131
6. Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004; 40: 1387- 1395
7. Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006; 118:1388-1393
8. Mofrad P, Contos MJ, Haque M et al. Clinical and histologic spectrum of non-alcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003; 37(6): 1286-1292
9. Dyson JK, Quentin M, McPherson et al. Nonalcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol 2014; 5:211-218
10. Tahan V, Canbakan B, Balchi H et al. Serum gamma-glutamyltranspeptidase distinguishes nonalcoholic fatty liver disease at high risk. Hepatogastroenterolgy.2008; 55:1433-1438
11. Nila Rafiq, Chunhong Bai, Yun Fang, Manirath Shrishord, Arthur Mccullough. Long-term follow-up with patients with Nonalcoholic fatty liver. Clinic Gastro and Hepatology 2009; 7:234-238
12. Esktedt M, Franzen LE, Mathiesen UL et al. Long term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006; 44:865-873
13. Adams LA, Lymp JF, St. Sauver J, et al. The natural history of non-alcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005; 129:113-121
14. Angulo P et al. Diagnosing steatohepatitis and predicting liver-related mortality in patients with NAFLD: two distinct concepts. Hepatology 2011; 53:1792-1794
15. Quentin M, Anstee, Robert D Goldin et al. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Path.2006: 87; 1- 6
16. Thomas C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systemic review. J. Hepatology 2012; 56: 255-266
17. Kesse-Guyot E, Ahluwalia N, Lassale C, Hercberg S et al. Adherence to mediterranean diet reduces the risk of metabolic syndrome: a 6-year prospective study. Nutr. Metab Cardiovasc Dis. 2013; 23:677-683
18. Gelli C, Tarocchi M, Abenavoli L, Galli A, De Lorenzo A et al. Effect of counseling-supported treatment with Mediterranean diet and physical activity on the severity of non-alcoholic fatty liver disease. World J. Gastro: 2017; 7:3150-3162
19. Ryan MC, Abbasi F, Lamendola C, Carter S et al. Serum ALT level decreases further with carbohydrate than fat restriction in insulin resistant adults. Diabetes care; 2007:30(5): 1075-1080
20. Haufe S, Engeli S, Kast P, Haas V et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology; 2011:53(5): 1504-1514
21. Liu F, Wang X, Wu G, Chen L et al. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: A meta-analysis. PLOS-ONE; 2015:11
22. Kennedy OJ, Roderick P, Buchanan R et al. Coffee including caffeinated and decaffeinated coffee, and the risk of HCC: A systematic review and dose-response meta-analysis. BMJ Open 2017; 7
23. Liu X, Lazeny AJ, Clements RH et al. Resolution of non-alcoholic steatohepatitis after gastric bypass surgery. Obese. Surg 2007; 17:486-492
24. Barker KB, Palekar NA, Browsers SP et al. Nonalcoholic steatohepatitis, effect of Roux-en-Y gastric bypass surgery. Am J Gastroenterol 2006; 101: 368- 373
25. Dixon JB, Bathal PS, Hughes HR et al. Nonalcoholic fatty liver disease: improvement in histological analysis with weight loss. Hepatology 2004; 39:1647-1654
26. Sanyal AJ, Chalasani N, Kowdley KV et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362:1675-1685
27. Islam M, Poly TN, Walther BA, Yang HC et al. Statin use and the risk of hepatocellular carcinoma: Ameta-analysis of observational studies. Cancers 2020; 12:671
28. Sigler MA, Congdon L, Edwars KL. An evidencebased review of statin use in patients with nonalcoholic fatty liver disease. Clinical medicine inshights Gastro 2017; 11:1-9
29. Ma SJ, Zeng YX, Zhou PC et al. Metformin use improves survival in diabetic liver cancer patients: systemic review and meta-analysis. Oncotarget 2016; 7:40
30. Armstrong MJ, Gaunt P, Aithal GP, Barton D et al. Liraglutide safety and efficacy in patients with nonalcoholic steatohepatitis (LEAN): a multicenter, double-blind, randomized, placebo-controlled phase II study. Lancet 2016; 13:387 (10019): 679-690
31. Newsome PN, Buchholtz K, Cusi K, Linder M et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. NEJM 2020 Mar 25; 384 (12): 1113-1124
32. Villar-Gomez E, Martinez-Perez Y, CalzadillaBertot L et al. Weight loss through life-style modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015 Aug; 149 (2): 367-378