Heart of Gold
Tanvir Rahman,Sara Abdelaziz,Reihaneh Moghadam,Vikram Agarwal,Craig Reiss
Department of Medicine, Division of Cardiology, St. Luke’s Hospital, Chesterfield, MO
Corresponding Authors: Tanvir Rahman
Correspondence: Instructor in Medicine, Washington University School of Medicine, Attending physician at the Barnes Jewish Hospital at Washington University in St. Louis, Missouri, Email: Tanvir.rahman@wustl.edu
Introduction
Eosinophilic granulomatosis with polyangiitis or EGPA (formerly known as Churg-Strauss syndrome) is a rare disorder with an estimated annual incidence of 1-3 per million. Cardiac involvement occurs in around 14% of the cases, which is a major cause of mortality [1]. Impaired left ventricular function, mild to severe valvular insufficiencies and pericardial effusions were reported in this patient population. Cardiac magnetic resonance imaging (MRI) is commonly used imaging technique to evaluate myocardial involvement which was noted to have poor sensitivity when compared to positron emission tomography (PET) scan [2]. We present this case to demonstrate the role of PET scan in diagnosis of this major cause of mortality in patients with EGPA.
Case report
A 64-year-old female with recently diagnosed EGPA characterized by asthma, chronic sinusitis, peripheral neuropathy, and absolute eosinophil count of 6.6 X 103 presented to her cardiologist's office complaining of episodic palpitations.
The symptoms were noted few times a week, lasting for a few seconds, associated with exertional shortness of breath but no chest discomfort, or syncope. Her medications at the time included prednisone 10 mg daily. An ambulatory cardiac monitor demonstrated non sustained (monomorphic) ventricular tachycardia (VT). 12-lead EKG showed sinus rhythm and non-specific T-wave abnormality. Transthoracic echocardiogram (TTE) revealed normal left ventricular cavity size, normal left ventricular systolic and diastolic function, and normal left ventricular mass index.
The left ventricular ejection fraction was measured at 66 %. No hemodynamically significant valvular abnormalities was seen on Doppler exam. Small pericardial effusion was noted. Cardiac magnetic resonance imaging (CMRI) failed to show any definitive localized late gadolinium enhancement (LGE) or myocardial edema but was suggestive of pericardial effusion (Fig.1a)
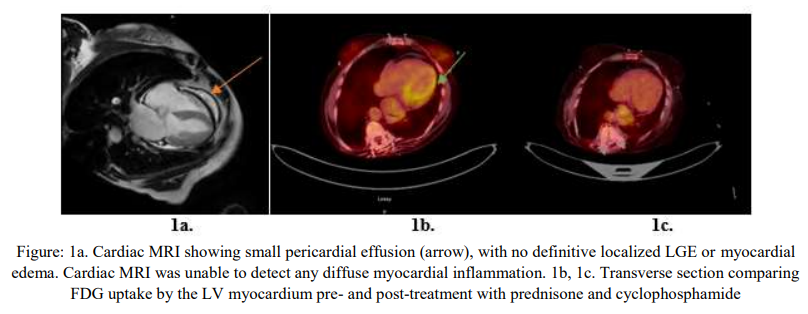
A myocardial PET scan was obtained and showed evidence of focal over diffuse Fluorodeoxyglucose (FDG) uptake by the left ventricular (LV) myocardium, with preserved perfusion, which was highly suggestive of early stages of myocardial inflammation (Fig.1b). She was started on a higher dose of prednisone, and cyclophosphamide by her Rheumatologist. Over the next 2 months, her palpitations improved. Three months after the initial PET scan, a repeat study did not show any active myocardial inflammation (Fig.1c), which correlated with symptomatic relief. She finished the cyclophosphamide regimen and was started on mepolizumab, an anti-interleukin 5 (IL-5) monoclonal antibody.
Discussion
EGPA is a multi-system disorder characterized by 3 phases. A prodromal phase which usually presents with atopic symptoms, allergic rhinitis and bronchial asthma. This is followed by eosinophilic acute phase, which includes peripheral blood eosinophilia and eosinophil-like infiltration of multiple organs, especially the lung and gastrointestinal tract. Finally, the vasculitic phase, which is a life-threatening systemic vasculitis of small vessels which commonly involves the heart [3]. Aggressive management with higher doses of corticosteroids, in addition to cyclophosphamide or azathioprine can result in resolution of pericardial effusions or LV dysfunction [4]. Traditionally, cardiac evaluation has included echocardiography, angiography, myocardial biopsy and cardiac MRI. In our case, PET scan facilitated early identification and treatment with a positive outcome.
1. CHURG J, STRAUSS L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol. 1951; 27 (2):277-301.
2. Marmursztejn J, Guillevin L, Trebossen R, Cohen P, Guilpain P, Pagnoux C, Mouthon L, Legmann P, Vignaux O, Duboc D. Churg-Strauss syndrome cardiac involvement evaluated by cardiac magnetic resonance imaging and positron-emission tomography: a prospective study on 20 patients. Rheumatology (Oxford). 2013 Apr; 52 (4):642-50. doi: 10.1093/rheumatology/kes155. Epub 2012 Jul 5. PMID: 22772324.
3. Antonio Greco, Maria Ida Rizzo, Armando De Virgilio, Andrea Gallo, Massimo Fusconi, Giovanni Ruoppolo, Giancarlo Altissimi, Marco De Vincentiis, Churg–Strauss syndrome, Autoimmunity Reviews, Volume 14, Issue 4, 2015, Pages 341-348.
4. Matthieu Groh, Christian Pagnoux, Chiara Baldini, Elisabeth Bel, Paolo Bottero, Vincent Cottin, Klaus Dalhoff, Bertrand Dunogué, Wolfgang Gross, Julia Holle, Marc Humbert, David Jayne, J. Charles Jennette, Romain Lazor, Alfred Mahr, Peter A. Merkel, Luc Mouthon, Renato Alberto Sinico, Ulrich Specks, Augusto Vaglio, Michael E. Wechsler, JeanFrançois Cordier, Loïc Guillevin, Eosinophilic granulomatosis with polyangiitis (Churg–Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management, European Journal of Internal Medicine, Volume 26, Issue 7, 2015, Pages 545-553
