Gaze Weakness Neglect and Speech (GWNS), An In-hospital Acute Ischemic Stroke Scale of Large Vessel Occlusion (LVO) for Faster Treatment: May be Utilized in The Developing Countries.
Yahia M Lodi, Adam Bowen, Aria Soltani, Irfan Khan, Heidi Hindsley, Anas Hourani
Upstate Medical University, Binghamton & NYUHS-Hospitals, Binghamton, NY;
Fort Hays State University, Haya, KS.
-------
Contact Information:
Yahia M Lodi, MD, FAHA, FANA, FAAN.
CAST Certified in Neuroendovasclar Surgery
Professor and Neurosciences Academic Chair
Neurology System Chair-UHS Hospitals.
Upstate Medical University, Binghamton, NY
Phone: 515-663-6020
Email: Yahia.lodi@gmail.com
Note: major contents of this manuscript have been published by the author in “Medical Research Achieve”, the official journal of ESMED.
Corresponding Authors: Yahia M Lodi MD, FAHA, FANA, FAAN.
Correspondence: CAST Certified in Neuroendovasclar Surgery, Professor and Neurosciences Academic Chair Neurology System Chair-UHS Hospitals.BACKGROUND
Despite the advancement in acute ischemic stroke with large vessel occlusion (LVO), golden time is lost in assessment lengthy neurological examination and redundantly in the Emergency department, often after emergency medical service prehospital stroke scale evaluation indicating possible LVO. A simple acute ischemic stroke scale (AISS) of the cortical representations of the anterior circulation can rapidly predict LVO, saving precious time to initiate early intravenous tissue plasminogen activator and endovascular mechanical thrombectomy. We proposed an ASIS in the emergency department called Gaze Weakness Neglect Speech (GWNS) stroke scale of LVO to evaluate its feasibility and predictability for the detection of LVO in anterior circulation in the emergency department. Additionally, to evaluate if time can be gained that has been lost in obtaining National Institute of Health stroke Scale (NIHSS) and computed tomographic angiography (CTA), avoiding unnecessary radiation. Finally, we are proposing that the GWNS stroke scale may open the opportunities in the developing countries, where the acute ischemic stroke system care pathway is weak.
METHODS
This is a prospective observational study. An institutional review board permission was obtained, and patient enrollment started in January 2020 and ended in January 2021. Consecutive patients from January 2020 to September 2021 were selected from the database. The GWNS stroke scale was used by stroke and vascular neurologist during the emergency triage. The GWNS stroke scale scores range from 0 to 4 (1 for positive 0 for negative). The GWNS stroke scale assesses gaze deviation or gaze preference (G), presence of any weakness (W), neglect/disregard (N), and any speech impairment (S). Demographic data, CTA/cerebral angiographic data, and scores from NIHSS were also collected. The collected data was analyzed by a biostatistician to determine the association between the GWNS scale score and LVO.
RESULTS
In our study,109 qualifying patients were selected. Fifty-eight patients had GWNS stroke scale score of 3 or 4, with 57 having confirmed LVO and 1 presenting after a seizure. The GWNS stroke score ≥3 (0.86) correlated with LVO better than NIHSS (0.67), regardless of hemisphere side involvement. The GWNS stroke scale score of ≥3 also was effective in detection of proximal and distal blood vessels occlusion in the anterior circulation (Internal carotid artery, middle cerebral artery and its branches). A GWNS stroke scale score of ≥3 with presence of gaze was the most predictive for LVO (0.9) followed by neglect/disregards (0.8). The time to obtain GWNS stroke scale was 1.5 minutes (range 1-3) and time to obtain/interpretation CTA was 41.3 +/- 7.4 minutes after emergency department arrival (range: 29-51 minutes).
Conclusions: Our Gaze Weakness Neglect Speech stroke scale can be performed rapidly in the emergency department and is highly predictive of LVO in the internal carotid artery, middle cerebral artery and middle cerebral branches. A GWNS stroke scale score of ≥3 is highly predictive of LVO, especially when gaze or neglect is present. Patients can potentially bypass CTA or advanced imaging in future studies, saving precious time and millions of brain cells for better outcome. This fast and simple AIS scale of LVO could be utilized in the developing countries including Bangladesh, where recourses are limited.
Gaze Weakness Neglect and Speech (GWNS), An In-hospital Acute Ischemic Stroke Scale of Large Vessel Occlusion (LVO) for Faster Treatment: May be Utilized in The Developing Countries.
Yahia M Lodi, Adam Bowen, Aria Soltani, Irfan Khan, Heidi Hindsley, Anas Hourani
Upstate Medical University, Binghamton & NYUHS-Hospitals, Binghamton, NY;
Fort Hays State University, Haya, KS.
-------
Contact Information:
Yahia M Lodi, MD, FAHA, FANA, FAAN.
CAST Certified in Neuroendovasclar Surgery
Professor and Neurosciences Academic Chair
Neurology System Chair-UHS Hospitals.
Upstate Medical University, Binghamton, NY
Phone: 515-663-6020
Email: Yahia.lodi@gmail.com
Note: major contents of this manuscript have been published by the author in “Medical Research Achieve”, the official journal of ESMED.
Corresponding Authors: Yahia M Lodi MD, FAHA, FANA, FAAN.
Correspondence: CAST Certified in Neuroendovasclar Surgery, Professor and Neurosciences Academic Chair Neurology System Chair-UHS Hospitals.
AIS: acute ischemic stroke
AISS: acute ischemic stroke scale
AHA: American Heart Association
ASPECT: Alberta Stroke Program Early CT Score
CPSS: Cincinnati Prehospital Stroke Scale
CIN: contrast-induced nephropathy
CTA: computed tomography angiography
DWI: Diffusion-weighted imaging
ED: emergency department
FAST-ED: Field Assessment Stroke Triage for Emergency Destination (FAST-ED)
GWNS: Gaze Weakness Neglect Speech
IVTPA: Intravenous Tissue Plasminogen Activator
LVO: Large Vessel Occlusion
mRS: Modified Rankin Scale
EVMT: Endovascular Mechanical Thrombectomy
VN: Vascular Neurologist
NIHSS: National Institutes of Health Stroke Scale
NPV: negative predictive value
PPV: positive predictive value
RACE: Rapid Arterial Occlusion Evaluation Scale
Introduction
In an acute ischemic stroke (AIS) approximately 1.9 million cells die in each minute of waiting. Patients with a large vessel occlusion (LVO) have a higher chance of morbidity and mortality compared with those without LVO.1 Additionally, approximately one third of patient with AIS may have LVO.1 The prestigious ground breaking randomized controlled trials (RCT)2-7 have demonstrated the highest level of evidence in medicine for the treatment and outcome of AIS with LVO up to 24 hours of symptoms and considered as a standard of care.8-10 The mortality and disabilities also have diminished significantly over the last 10 years as AIS with LVO are receiving endovascular mechanical thrombectomy (EVMT) in the extended period of time, especially after the results of previously mentioned positive clinical trials. State, local and national leadership organizations also embraced and acknowledged the need for stroke system care. Therefore, faster clinical detection of AIS patients with LVO and providing early perfusion therapy with EVMT may potentially continue to improve outcome. The more inclusive definition of large vessel occlusions (LVO) for the anterior circulation (AC), defined as a blockage to the International Carotid Artery Terminus (ICA-T) Middle cerebral artery (MCA) proximal (MCA-M1), MCA bifurcation (MCA-M2), MCA trifurcation (MCA-M3) including ICA origin occlusion with MCA thrombus, causes approximately a third of acute ischemic stroke (AIS). It is recommended to have door-to-needle time for intravenous thrombolytic to be under 60 minutes, preferably in 30 minutes and groin puncture for EVMT in 60 minutes who was last known normal within 4.5 hours.9 Brain imaging is recommended within 20 minutes, consisting of computed tomography (CT) and CTA/CT perfusion (CTP)/diffusion weighted imaging (DWI) may require if EVMT criteria are met.6,7 The highest recommendation of ET criteria recommends a patient: have a pre-stroke modified Rankin score (mRS) of 0-1; have a causative LVO, be over 18 years old, have a National Institutes of Health Stroke Scale (NIHSS) of ≥6; have an Alberta Stroke Program Early CT Score (ASPECTs) of ≥6; have groin access within 6 hours of onset.4 The DAWN and DUFUSE criteria of clinical imaging mismatch consider penumbra and NIHSS to further expand ET to 24 hours in select patients.6,7
Even with15 minute delay in EVMT in the triage of the emergency department (ED) can significantly affect the functional outcomes for 64 out of 1000 individuals.11 Five trials have demonstrated that both intravenous activated tissue plasminator activator (IVTPA) and EVMT are significantly linked to better outcomes with each hour saved, resulting in improved modified Rankin scale (mRS) in 90 days and greater functional independence.2-7 In thrombolytic found to be almost 4 times more effective at 1 hour than at 3 hours.12
Prehospital screens have been used to expedite EVMT for LVO by bringing patients to facilities with EVMT capabilities. The validated prehospital screens include the Cincinnati Prehospital Stroke Scale (CPSS), the Field Assessment Stroke Triage for Emergency Destination (FAST-ED), and Rapid Arterial Occlusion Evaluation (RACE).13 These screens requisite confirmatory imaging with CTA for definitive LVO diagnosis. Additionally, many stroke patients arrive at ER without ambulance and without pre-hospital LVO screening. Nonetheless, the NIHSS is still done by a doctor trained in stroke care in the hospital setting as required by the stroke accreditation organizations further delaying EVMT and creating redundancy in LVO evaluation.14,15 However, these validated tests have repeatedly shown that they are equal or superior to the NIHSS at predicting LVO, while being shorter and more straightforward.13-15 There are limitations of NIHSS and other prehospital scales in identifying LVO in the posterior circulation, which can limit EVMT candidacy.
Current American Heart Association (AHA) guidelines provide 1a evidence for thrombectomy in patients with a NIHSS score of ≥ 6 with corroborating imaging.13 The time to perform and evaluate these tests significantly delays EVMT. A hospital test that can circumvent the need for a CTA, NIHSS evaluation, and is more predictive of LVO could significantly improve patient outcomes. We absolutely agree that all stroke patients deserve a comprehensive neurological evaluation using an NIHSS. However, its utilization in the setting of AIS with LVO detection and treatment may potentially delay and miss crucial cortical signs that are not included in NIHSS. Our study evaluates the use of a proposed AISS in the ED/hospital called GWNS, which rapidly identifies LVO. The GWNS scale of LVO is a simple AISS of cortical representations of the AC that is designed to rapidly predict LVO so that EVMT for early perfusion therapy can be initiated. This study evaluates the feasibility and predictivity of the GWNS stroke scale by comparing it to the NIHSS in identifying LVO like an electrocardiography (EKG) in acute myocardial infarction (AMI). Authors would like to evaluate if precious time and radiation could have been saved if GWNS stroke scale would have been used EVMT for prefusion therapy without obtaining CTA. Authors also believe that the GWNS stroke scale of LVO may serve as an initial tool in the developing countries including Bangladesh for early evaluation and treatment, where resources and stroke-care pathways are limited.
Methods
This is a prospective observation study on acute ischemic stroke. Institutional review board approval was obtained to begin enrolling patients in January 2020, and enrollment is ongoing. Consecutive patients with AIS with the suspicion of LVO were selected from January 2020 to September 2021. All patients received an initial NIHSS from the triaging physician. Additionally, the GWNS scale was also performed by the triaging vascular neurologist involved in the GWNS clinical study. As part of the protocols, during triage, all patients with NIHSS 6 or higher and/or suspicion of LVO including presence of gaze or neglect, received CTA head and neck in our study. Patient with LVO based on the CTA underwent MT. Patient all data including time of onset or last known normal, presenting ASPECT on CT head, CTA results, time to perform GWNS scale, time to perform CTA with interpretation were collected prospectively. Data was analyzed by a biostatistician using Minitab statistical software in the medical field to determine the association of NIHSS and GWNS stroke scale score with the presence of LVO.
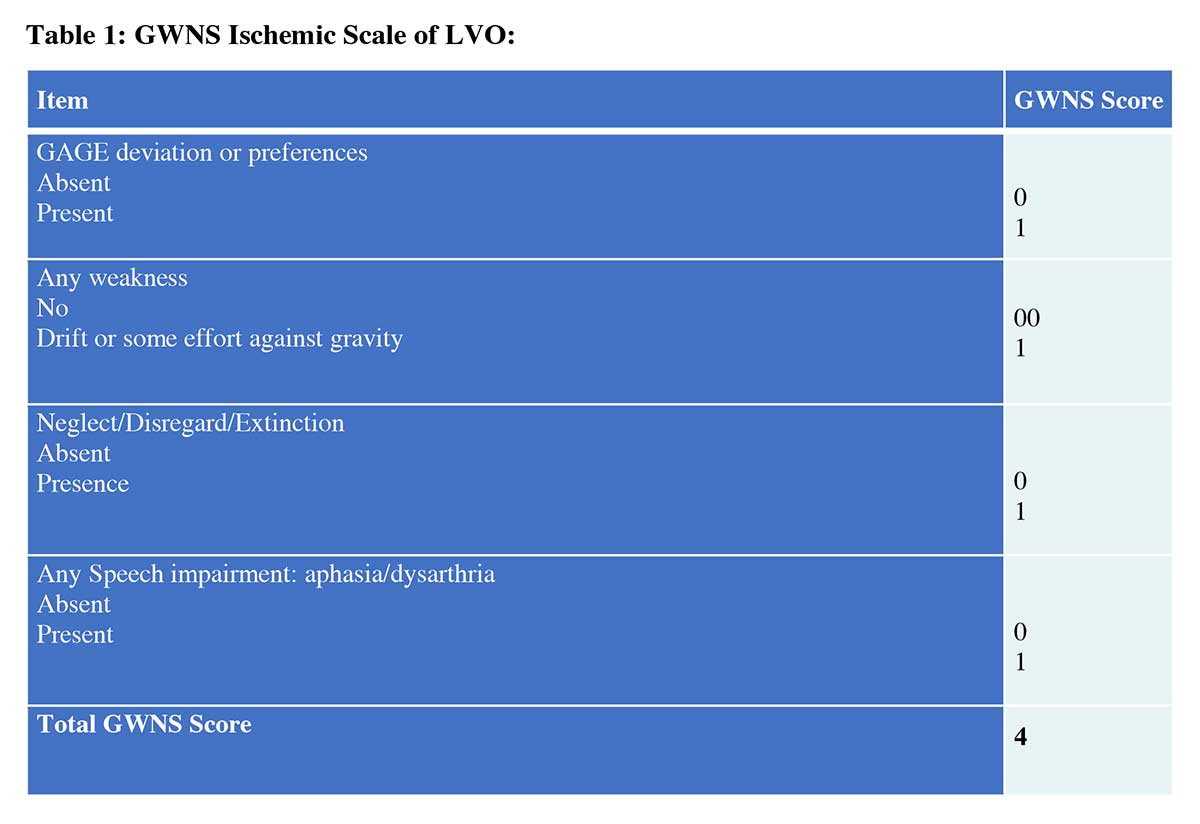
The GWNS stroke scale (Table 1) is a binary scale scored either 0 or 1 for a range of 0-4 points, with 0 being negative and 1 being positive. Gaze is positive if gaze deviation/preference or subtle gaze is present. Weakness is positive if there is any muscular weakness present. Neglect is positive if there is any neglect or disregard present. Speech is positive if there is any aphasia or dysarthria present. GWNS was utilized by a stroke fellowship-trained vascular neurologist during triage in the emergency department (ED).
Instructions of performing GWNS stroke scale of LVO in the hospital:
- Gaze: Stand in front of the patient and observe if patient’s head is straight and eyes are looking forward or deviating to the left or right. Ask the patient, “can you look at me?” If the patient is unable to look at you/correct the gaze from the forcefully deviated to the right or to the left side, it is called “Gaze deviation”. If patient can fully or partially correct the gaze and look at you but drift back to previous position, it is called “Gaze preference”. If the patient can fully correct gaze and look at the left or wright without drifting back during interaction, but eyes drift back to the site of ictus when you are not engaging the patient, it is defined as “subtle Gaze”.
- Weakness: Positive (1) if there is any weakness of face and/or limbs, from subtle drift to full paralysis. Ask the patient to “smile” or “hold up your arms”.
- Neglect: Positive (1) if any visual or tactile neglect, disregard or extinction, regardless of the severity. Neglect usually present in the right hemispheric or non-dominant stroke. Ask the patient to show each arm. If the patient shows only right arm when asked to show left, it is called neglect or disregard. In severe neglect, patients will not even recognize their own affected arm. Subtle neglect example could be when patient asked to close their eyes and they were touched in their both sides simultaneously, they will only recognize being touched to the unaffected side. Visual neglect could include patient unable to see the examiners hands moving on one side during simultaneous visual field testing.
- Speech: Positive (1) if there is any slurred speech, inability to understand, inability to respond, inability to repeat, inability to object name or paraphasic errors. If any are present, this is considered positive and do not need to test all components. Ask patient "tell me your name". Ask patient to repeat "today is a sunny day". Show them a pen and ask "what is this?". Paraphasic error example could be response of "Ben" instead of "Pen". In case of a global aphasia, patient will not be able to do any of the above, which sometimes mistakenly called “patient is mute” instead of having sever aphasia.
Instruction how to perform the Alberta Stroke Program Early CT Score (ASPECTS):
The Alberta Stroke Program Early CT Score (ASPECTS) is a quantitative scoring tool used to evaluate early ischemic change on head CT and can predict functional outcome despite thrombolytic therapy. A full score of 10 indicates a normal CT without any evidence of early ischemic change whereas a score of 0 indicates diffuse MCA area involvement. Each affected radiographic area subtracts 1 from the total score, and the overall score correlates inversely to the severity of stroke. The 10 regions involved in this calculation are caudate, internal capsule, lentiform nucleus, insular ribbon, and M1-M6 areas.
- Caudate = Involved (-1), Not involved (0)
- Internal capsule = Involved (-1), Not involved (0)
- Lentiform nucleus = Involved (-1), Not involved (0)
- Insular ribbon = Involved (-1), Not involved (0)
- Anterior MCA cortex (M1) = Involved (-1), Not involved (0)
- MCA cortex lateral to insular ribbon (M2) = Involved (-1), Not involved (0)
- Posterior MCA cortex (M3) = Involved (-1), Not involved (0)
- Anterior cortex rostral to M1 (M4) = Involved (-1), Not involved (0)
- Lateral cortex rostral to M3 (M5) = Involved (-1), Not involved (0)
- Posterior cortex rostral to M3 (M6) = Involved (-1), Not involved (0)
Results (ASPECTS 0-10)
- ASPECTS 10 = normal
- ASPECTS ≤7 = correlation with negative functional outcome using mRS
- APSECTS 0 = diffuse MCA territory involvement
Pexman, J. H., Barber, P. A., Hill, M. D., Sevick, R. J., Demchuk, A. M., Hudon, M. E., Hu, W. Y., & Buchan, A. M. (2001). Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR. American journal of neuroradiology, 22(8), 1534–1542.
Statistical analysis
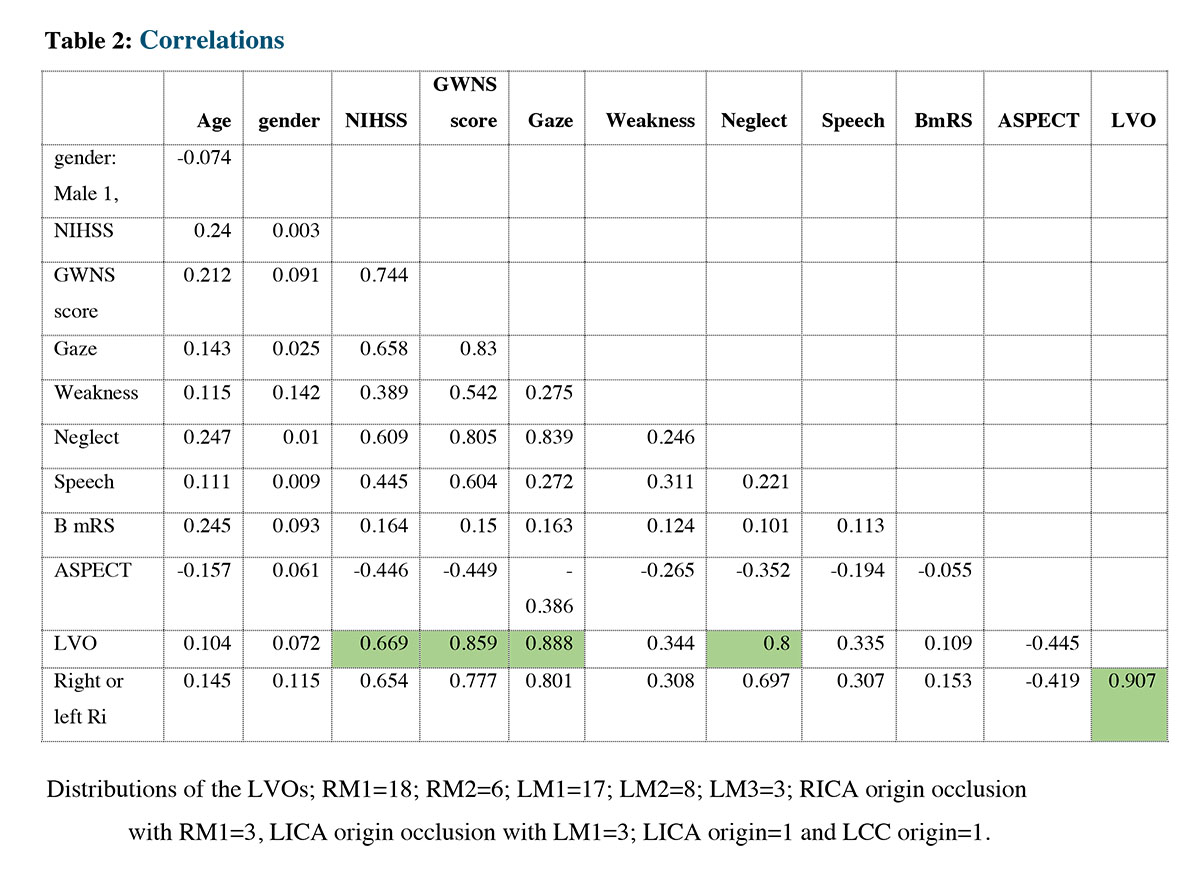
The correlation coefficient is a statistical measure that indicates the strength and direction of the relationship between two variables. Correlation is measured on a scale from -1 to 1, where -1 indicates a perfect negative correlation, 0 indicates no correlation, and 1 indicates a perfect positive correlation. In this case, we are interested in the correlation between LVO (large vessel occlusion) and several other variables: NIHSS (National Institutes of Health Stroke Scale), GWNS (Gaze, Weakness, Neglect, Speech), Gaze, and Neglect. The correlation between LVO and NIHSS is 0.669 (0.67), which indicates a moderately positive relationship between the two variables. The correlation between LVO and GWNS stroke scale score is 0.859 (0.86), indicating a strong positive relationship. The correlation between LVO and Gaze is 0.888 (0.9), which suggests a very strong positive relationship between the two variables. Finally, the correlation between LVO and Neglect is 0.8, indicating a strong positive relationship. These correlation coefficients table shows that the NIHSS, GWNS, GAZE, and Neglect are predictors to the LVO because of the strength relationship between them.
Results
In our study 109 qualifying patients were selected (52% women). In the ED, the presenting mean age was 70.32 +/- 15 yrs. The median baseline GWNS was 3 (range 1-4), median NIHSS was 12 (range 1-27), median ASPECT was 9 (range 4-10) in those who had CTA during triage based on suspected LVO. In our prospective cohort, LVO was detected in 44% of qualifying patients.
In our prospective cohort, 58 patients had GWNS stroke scale score of 3 or 4, with 57 having confirmed LVO accept one who presented after a seizure. Of the confirmed LVO patients, 57 had either gaze deviation or preference, as well as weakness and/or speech impairment. However, only 46 had demonstrated neglect at presentation. Neglect was linked to right hemispheric LVO in all patients. No patient with only weakness and/or speech impairment had LVO if they lacked gaze and neglect, which is a very important finding in our study. The GWNS stroke scale score predicted LVO better (0.85) than NIHSS (0.67), regardless of hemisphere side involvement. A GWNS stroke scale score of ≥3 with gaze was the most predictive for LVO (0.9) followed by presence of neglect (0.8). (Table 2)
Instruction how to perform the Alberta Stroke Program Early CT Score (ASPECTS):
The Alberta Stroke Program Early CT Score (ASPECTS) is a quantitative scoring tool used to evaluate early ischemic change on head CT and can predict functional outcome despite thrombolytic therapy. A full score of 10 indicates a normal CT without any evidence of early ischemic change whereas a score of 0 indicates diffuse MCA area involvement. Each affected radiographic area subtracts 1 from the total score, and the overall score correlates inversely to the severity of stroke. The 10 regions involved in this calculation are caudate, internal capsule, lentiform nucleus, insular ribbon, and M1-M6 areas.
- Caudate = Involved (-1), Not involved (0)
- Internal capsule = Involved (-1), Not involved (0)
- Lentiform nucleus = Involved (-1), Not involved (0)
- Insular ribbon = Involved (-1), Not involved (0)
- Anterior MCA cortex (M1) = Involved (-1), Not involved (0)
- MCA cortex lateral to insular ribbon (M2) = Involved (-1), Not involved (0)
- Posterior MCA cortex (M3) = Involved (-1), Not involved (0)
- Anterior cortex rostral to M1 (M4) = Involved (-1), Not involved (0)
- Lateral cortex rostral to M3 (M5) = Involved (-1), Not involved (0)
- Posterior cortex rostral to M3 (M6) = Involved (-1), Not involved (0)
Results (ASPECTS 0-10)
- ASPECTS 10 = normal
- ASPECTS ≤7 = correlation with negative functional outcome using mRS
- APSECTS 0 = diffuse MCA territory involvement
Pexman, J. H., Barber, P. A., Hill, M. D., Sevick, R. J., Demchuk, A. M., Hudon, M. E., Hu, W. Y., & Buchan, A. M. (2001). Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR. American journal of neuroradiology, 22(8), 1534–1542.
Statistical analysis
The correlation coefficient is a statistical measure that indicates the strength and direction of the relationship between two variables. Correlation is measured on a scale from -1 to 1, where -1 indicates a perfect negative correlation, 0 indicates no correlation, and 1 indicates a perfect positive correlation. In this case, we are interested in the correlation between LVO (large vessel occlusion) and several other variables: NIHSS (National Institutes of Health Stroke Scale), GWNS (Gaze, Weakness, Neglect, Speech), Gaze, and Neglect. The correlation between LVO and NIHSS is 0.669 (0.67), which indicates a moderately positive relationship between the two variables. The correlation between LVO and GWNS stroke scale score is 0.859 (0.86), indicating a strong positive relationship. The correlation between LVO and Gaze is 0.888 (0.9), which suggests a very strong positive relationship between the two variables. Finally, the correlation between LVO and Neglect is 0.8, indicating a strong positive relationship. These correlation coefficients table shows that the NIHSS, GWNS, GAZE, and Neglect are predictors to the LVO because of the strength relationship between them.
Results
In our study 109 qualifying patients were selected (52% women). In the ED, the presenting mean age was 70.32 +/- 15 yrs. The median baseline GWNS was 3 (range 1-4), median NIHSS was 12 (range 1-27), median ASPECT was 9 (range 4-10) in those who had CTA during triage based on suspected LVO. In our prospective cohort, LVO was detected in 44% of qualifying patients.
In our prospective cohort, 58 patients had GWNS stroke scale score of 3 or 4, with 57 having confirmed LVO accept one who presented after a seizure. Of the confirmed LVO patients, 57 had either gaze deviation or preference, as well as weakness and/or speech impairment. However, only 46 had demonstrated neglect at presentation. Neglect was linked to right hemispheric LVO in all patients. No patient with only weakness and/or speech impairment had LVO if they lacked gaze and neglect, which is a very important finding in our study. The GWNS stroke scale score predicted LVO better (0.85) than NIHSS (0.67), regardless of hemisphere side involvement. A GWNS stroke scale score of ≥3 with gaze was the most predictive for LVO (0.9) followed by presence of neglect (0.8). (Table 2)
The time to obtain GWNS stroke scale was 1.5 minutes (range 1-3) and time to obtain with interpretation of CTA was 41.3 +/- 7.4 minutes after ED arrival (range: 29-51 minutes). We are presenting 3 cases who were enrolled using GWNS stroke scale and underwent EVMT.
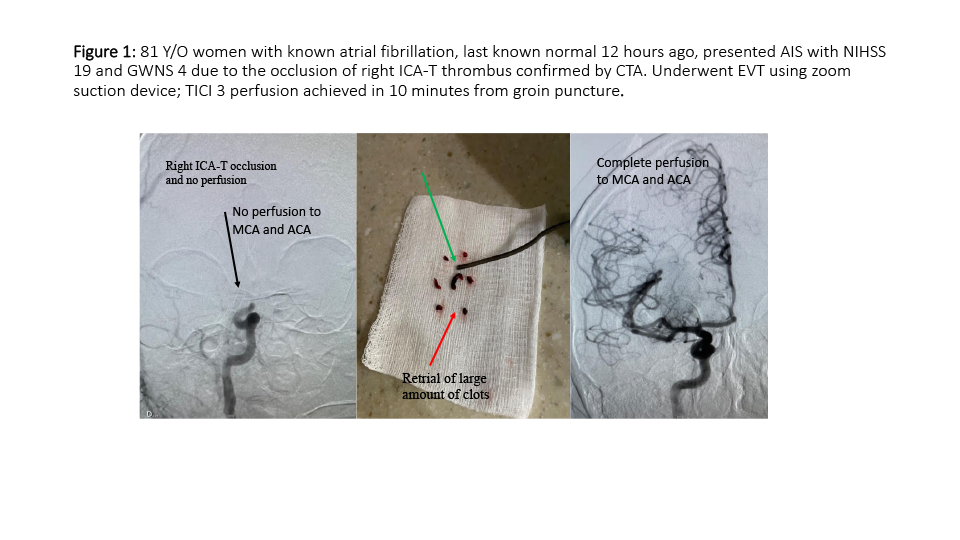
First case (Figure 1); 81 Y/O women last known normal 12 hours ago, presented AIS with NIHSS 19 and GWNS stroke scale score of 4 due to the occlusion of right ICA-T thrombus confirmed by CTA. Patient Underwent EVMT using zoom suction device; TICI 3 perfusion achieved in 10 minutes from groin puncture.
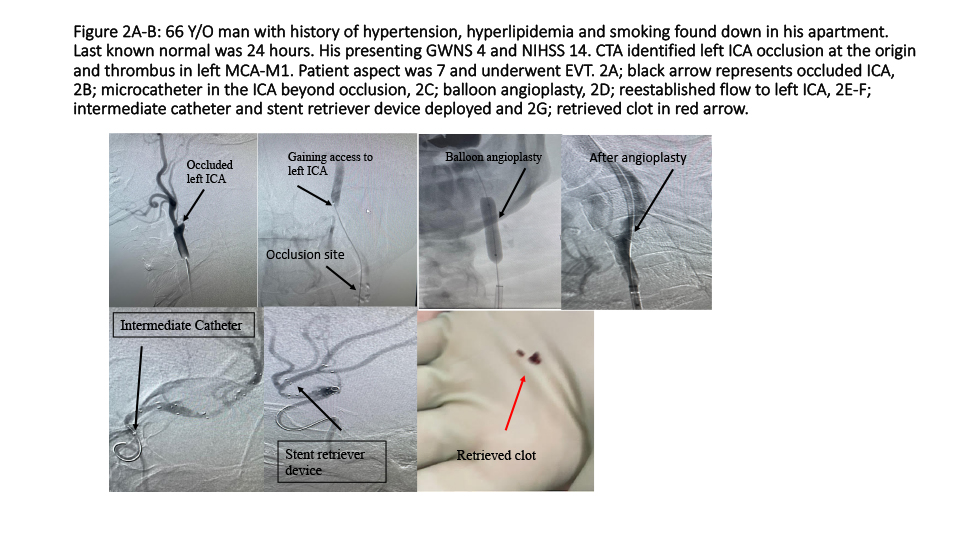
Second case (Figure 2A-B); 66 Y/O man with history of hypertension, hyperlipidemia and smoking found down in his apartment. Last known normal was 24 hours. Presentation GWNS stroke scale score was 3 and NIHSS 14. CTA identified left ICA occlusion at the origin and thrombus in left MCA-M1. Patient aspect was 7 and underwent EVMT. He received 300 mg rectal aspirin, and his blood pressure was kept 140 mm Hg to 180 mm Hg until ICA was revascularized. Blood pressure was brought down close to 140 mm Hg or lower during and after retrieval of clot from MCA. Patient required 3 balloon angioplasties before advancing balloon guide to the ICA. An intermediate catheter along with a stent retriever device was used. Inflation of balloon guide and suction through the intermediate catheter and balloon guide were obtained using trap technique resulting in complete perfusion.
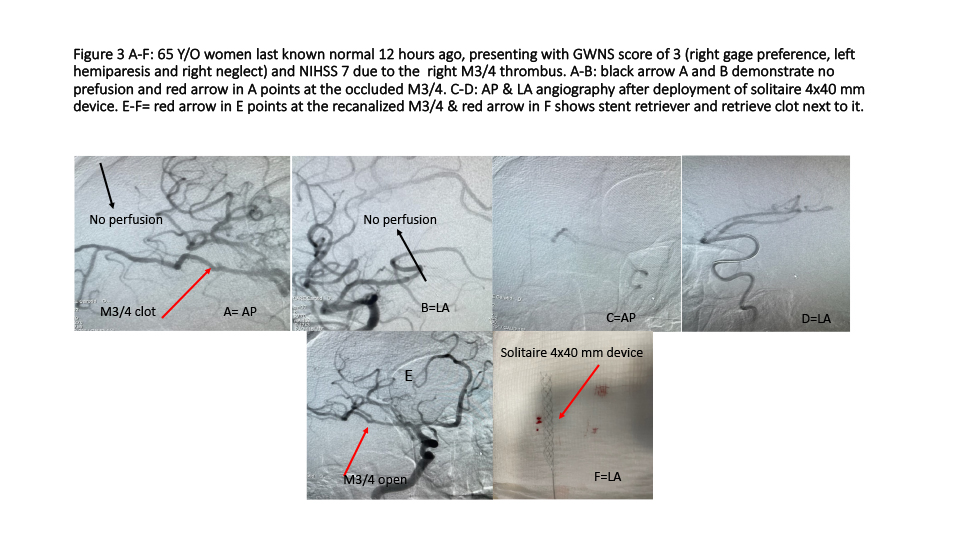
Third case (Figure 3 A-F): 65 Y/O women last known normal 12 hours ago, presenting with GWNS stroke scale score of 3 (right gage preference, left hemiparesis and right neglect) and NIHSS 7 due to the right M3/4 thrombus, underwent EVMT using similar strategies as described in 2nd case.
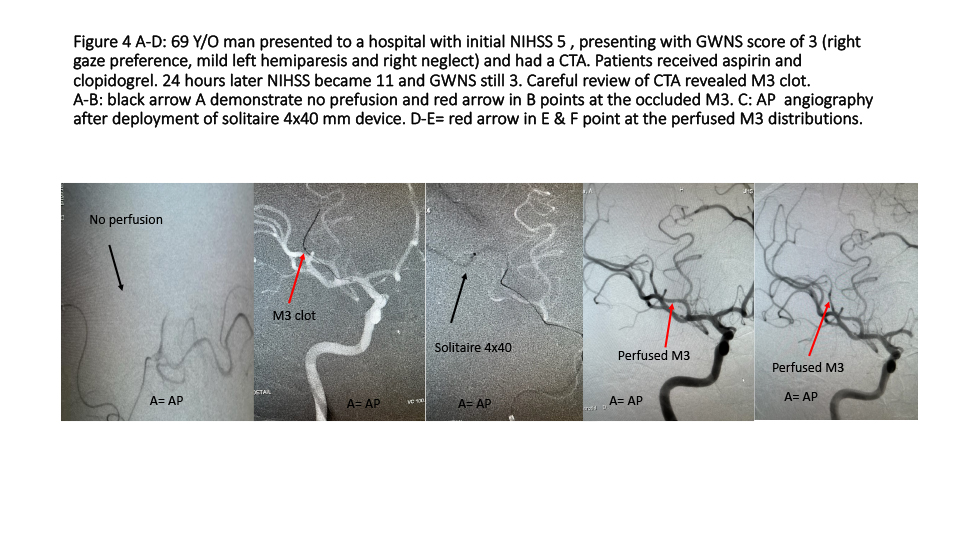
Patient number 4 (Figure 4 A-D): 69 Y/O man presented to a stroke center 24 hours ago with initial NIHSS 7 and GWNS stroke scale score of 3 (right gage preference, mild left hemiparesis and right neglect). Patients received aspirin and clopidogrel including standard treatment. 24 hours later, no improvement but NIHSS 11 now and still GWNS 3. A careful review of CTA demonstrated right M3 thrombus. Patient was transferred to our hospital and underwent perfusion therapy using EVMT. Patient was discharged home in 48 hours with NIHSS 0.
Discussion
This is the first in hospital/ER AISS of LVO in our knowledge that could be performed in few minutes and highly predictive of LVO. We found that GWNS scale score ≥3 was associated with LVO outperforming NIHSS irrespective of the side of lesion. Our GWNS scale score ≥3 with the presence of gaze predicted the highest in the detection of LVO followed by presence of neglect/disregards. Therefore, faster triage and treatment of patients with GWNS scale may potentially streamline ER stroke care for LVO with improved outcome (Table 2). Statistical measures of scales for LVO must be considered in the context of how quickly a patient was presented. However, our test was directly compared against a NIHSS control, outperforming it in ways that previous.13-15 However, true comparison is not possible as our GWNS is in hospital and others are prehospital.
If we look at all positive trials2-7 that leading to the EVMT as a standard of care in extended time.8-10 Despite the benefit in the trials2-7 a large number of patients treated with EVMT were not benefited despite the perfusion therapy. In MRCLEAN trial,2 patients with AIS with LVO were enrolled either in the IVTPA alone arm or with EVMT within 6 hours of symptoms onset. Functional outcome (mRS <2) with IVTPA and EVMT arm was 32% compared to 19% in the IVTPA alone group, meaning despite benefit, 68% of the EMVT arm did not achieve good functional outcome. In EXTENT-IA trial;3 70 patients in 14 centers in Australia and New Zealand received IVTP within 4.5 hours were randomized within six hours after CT, CTA, and CTP (CT perfusion).
Recanalization in IVTPA plus Endovascular arm was 100% compared to IVTPA alone at 37%. Seventy one percent in the combined group achieved good functional outcome (mRS<2) compared to only 40% in IVTPA alone, indication 29% in the combined arm didn’t achieve good outcome. In the ESCAPE trial,4 patients were selected based on CT head with ASPECT > 6 and LVO on CTA. Good functional outcome (mRS<2) was observed to be 53% in Endovascular arm compared to 29% in the IVTPA arm, reflecting that 47% with EVMT had poor outcome based on the definition. Similarly, in the REVASCAT trial;5 patients enrolled in EVMT arm demonstrated good functional outcome of 43.7% compared to 28.2% in IVTPA arm, meaning 56.3% in EVMT arm had poor functional outcome. The trial that have enrolled patients up to 24 hours as a late arrival (DEFUSE, DAWN) also have demonstrated that despite good perfusion not all achieved good outcome. In the DEFUSE 3 trial,6 182 patients with pre-stroke modified Rankins score of 0-2, presenting NIHSS > 6 were enrolled for 6 to 16 hours groin puncher for EVMT using diffusion perfusion mismatch (RAPID software) of 1.8 or more. Recanalization in the endovascular arm was 78% compared to only 18% in medical arm. Good functional outcome (mRS<2) was 45% in Endovascular arm compared to the medical arm 17%. Additionally, mortality was only 14% in the endovascular arm compared to 26% in the medical arm. Both good outcomes and mortality were significantly better in Endovascular arm compared to the medical arm. The number needed to treat for positive benefit was 2.0, OR 2.8 (1.6-4.7), p<0.0001, Adjusted OR 3.4 (2.0-5.8), p<0.0004). This was a revolutionary trial which demonstrated positive results for the late arrival of AIS with LVO. If we look carefully, in DUFUSE trial, 55% patients in the EVMT did not achieved good functional outcome. The DAWN trial7 went further to enroll patients for EVMT within 24 hours including wakeup strokes. This was the final game changer trial for AIS with LVO that gave a solid platform for treatment of late arrival of stroke patients. The 90 days functional outcome (mRS<2) was 49% in endovascular arm compared to only 13% in the medical arm, again demonstrating that despite excellent results, 51% patients in EVMT arm didn’t have good clinical outcome. Hypothetically speaking, above gold standard studies with positive results have used traditional triage/transfer and evaluation using NIHSS and have used advanced imaging of CT/CTA/CTP/DWI to confirm LVO and selection of patients requiring longer time that might be responsible for loosing ischemic penumbra and not achieving good function in a large number of patients. However, the ischemic penumbra is dynamic and multiple factors are responsible to keep the penumbra alive; presence of collaterals, age, presenting blood pressure and individual tolerability of ischemia for the brain tissue. Hypothetically speaking, if patients are selected with faster AIS with LVO scale and received early perfusion therapy, it is likely that more patients, could have been benefited with perfusion therapy in the trials. However, one cannot definitely depict the outcome at it is multifactorial and it is not the scope in our study.
Our GWNS stroke scale also was significantly faster than the hospital performed NIHSS. The time required to obtain a CTA with interpretation of our study raises questions about its necessity, with GWNS stroke scale score correctly identifying LVO in 57 out of 58 patients before imaging. In those patients that do not meet the eligibility criteria for ET, CTA is used to provide information on patient eligibility without an evaluation of creatinine, creating the potential for further harm.8,16 With a median time of 41 minutes for a CTA with interpretation, significant time is being lost. Bypassing CTA and using non-contrast CT with GWNS stroke scale uses fewer hospital resources and avoids additional harm from exposure of radiation and contrast. In addition, some patients might be precluded from CTA based on preexisting renal impairments. Others might be tested for creatinine before CTA, if physicians are not adhering to evidence-based practices, further delaying EVMT in both scenarios. Although the incidence of contrast-induced nephropathy (CIN) in those patients without risk factors is low, CIN can be associated with increased morbidity.16,17 These added from CIN, preclusion from EVMT, delays in assessment of creatinine all have the possibility to greatly impact patient outcomes.
The GWNS stroke scale gives precedence to the major anterior cortical objective signs, significantly simplifying and shortening the triage process. The GWNS stroke scale is different from prehospital and NIHSS exams, as it gives equal importance to all four of its elements, without differentiating them based on severity (Table 1). Many components of NIHSS do not represent cortical sign, subjective and more importantly, gaze and neglect are not a part of NIHSS. Our GWNS stroke scale employs broader and simpler language compared to other LVO scales. For example, weakness is defined as any present weakness, gaze is equivalent to preference, neglect includes any sensory modalities and is equivalent to disregard, and speech is any speech deficit and is equivalent to dysarthria. In direct comparison, NIHSS is very bloated for LVO detection. It contains many points that are not represented in cortical strokes, which are the main territories affected by LVO. The ≥6 cut off for EVMT would not be met by NIHSS if, for example, someone had a left arm motor drift (1 point), mild aphasia (1 point), and visual inattention/neglect (1 point). This same patient would score 3 in GWNS stroke scale and would be in the group found to be the most predictive for LVO (ROC 0.9) in our study.
While the prehospital and NIHSS scores have their merits, they also have limitations that could affect patient access to ET and IVTPA. The NIHSS and prehospital scales are limited in their ability to fully encompass LVO. The NIHSS tends to do well at identifying anterior LVO in the first 6 hours, with a peak positive predictive value (PPV) of 86.4%, a score of 9 at 3 hours, and 84.4% with a score of 7 at 6 hours.13,18 Kesinger et al. demonstrated a lower sensitivity at 52% and a high specificity with the NIHSS. However, the NIHSS tends to poorly identify posterior LVO. Its PPV tends to deteriorate over time, especially evaluating for LVO after 6 hours.19 The NIHSS is lengthy, time-consuming, and requires a steeper learning curve for training, resulting in a greater potential for scoring variability, especially in fast-paced environments like the ED15 GWNS stroke scale, by comparison, has a shallow learning curve.
Prehospital stroke scales for LVO have significant advantages over the NIHSS, being quicker and less complex to perform. However, the studies that have validated these scales have limitations, and there is heterogeneity in the methods used to evaluate their efficacy (20). Specifically, many of these studies have varying definitions of LVO, with some including the posterior circulation and others not.13,14,20
The FAST-ED scale uses a point system similar to the NIHSS but assigns fewer points for facial palsy, arm weakness, and severe speech changes and it assigns equal points for eye deviation that differentiates between partial and forced, and denial and neglect.13 Lima et al. analyzed the FAST-ED in the hospital setting and found it comparably accurate to the NIHSS and more accurate than RACE and CPSS with statistical significance in the latter two (FAST-ED: .81, NIHSS: .90, RACE .77, CPS: .75).13 Their study also found that a FAST-ED score of 4 or greater had a similar specificity and sensitivity to a NIHSS score of 10 or greater. Like the GNWS, FAST-ED can quickly detect and rule out LVO with just a few point differences. However, FAST-ED is not superior to NIHSS in its ability to predict LVO. The RACE scale has good sensitivity and specificity in field has limitations in LVO diagnosis.14 Its validity was tested using transcranial Doppler, which is not the standard of care.8 RACE gives greater weight to motor symptoms, making it less effective in distinguishing between small vessel occlusions (SVO) and LVO, as motor symptoms can also indicate subcortical strokes. It only assigns one point for gaze deviation, which GWNS showed to be highly predictive of LVO. RACE also narrows its definition of aphasia to only comprehensive aphasia, missing the other aphasias associated with LVO. RACE’s point system gives greater weight to right-hemisphere symptoms, but these symptoms are known to have lower PPV have lower PPV.14 RACE performs similar to NIHSS for LVO, like FAST-ED. RACE of ≥6 has a sensitivity of .85 and specificity of .68, and PPV of .42, negative predictive value (NPV) of .94 in detecting LVO.
As we mentioned before, a true comparison of our GWNS stroke scale with FAST-ED or RACE is not scientific. Because GWNS stroke scale in an in hospital/ER AISS of LVO and FAST-ED and RACE are field/pre-hospital AIS of LVO. Both performed by EMS personnel and based on the suspicion score of LVO, patients are bought to the thrombectomy capable or comprehensive stroke center ER for further evaluations with NIHSS followed by CT and CTA leading to confirm LVO and ET, which potentially may delay the perfusion therapy. On the other hand, GWNS stroke scale directly evaluates all patients in the ER in less than 3 minutes and highly predicts LVO if score ≥3. Interestingly, in our study, if GWNS stroke scale score was less than 3, none of the patients had LVO confirmed by CTA, making GWNS stroke scale score ≥3 a very reliable predictor of LVO. Therefore, an AIS patient with GWNS stroke scale score ≥3 with an acceptable ASPECT score can directly be taken for EVT with or without thrombolytic based on the eligibility criteria, cutting down time and avoiding radiation and contrast (Table 3). The author had a pilot trial21 in which patients were directly taken to EVMT without thrombolytic with 3 hours of symptoms onset and have demonstrated feasibility and good outcome.21 Most recent trials22,23 have demonstrated that the driver of good outcome in AIS with LVO is early perfusion with EVMT;22,23 patient who underwent EVMT without intravenous thrombolytic have similar good functional outcome comparted with those received thrombolytic before EVMT. We have initiated GWNS direct study in which patients will be randomized either directly to EVMT if patients GWNS stroke scale score ≥3 after a plain CT or CT and CTA to confirm LVO followed by endovascular mechanical thrombectomy to evaluate that if the GWNS-direct arm is associated with early perfusion and good outcome.
Limitations of study
Previous studies by Heldner and Kesinger have shown that AISS tends to have decreasing PPV as time passes in cases of true LVO.18,19 Our study, conducted in a hospital setting, may have higher predictivity due to being performed later in the patient course. However, comparing it directly to the NIHSS controls for this effect. The GWNS stroke scale was performed by a trained vascular neurologist, and its efficacy should be evaluated by other professionals. The study was conducted in a single center that may have longer transport times due to its geographic location, potentially increasing the accuracy of any AISS due to delays in patient presentation.18 Additionally, GWNS stroke scale is only implacable for LVO in the anterior circulation, not for the posterior circulation.
Future implications
Other studies have shown high agreement with the NIHSS in both prehospital and hospital settings, indicating that a simpler AISS like GWNS stroke scale could be easily used by other providers in the ED for quicker and more continuous care. While the GWNS stroke scale was evaluated by a trained vascular neurologist, it needs further evaluation among providers in both the prehospital and hospital setting. Other studies have found success with EVMT outside of NIHSS <6, suggesting the potential use of GWNS stroke scale without a NIHSS score.24 Further Studies are needed to explore the potential benefits of bypassing CTA in patients with GWNS stroke scale of ≥3. The use of GWNS stroke scale in other hospital systems and different geographic areas can add validity to its hospital use. Direct comparisons with other prehospital scores in the prehospital setting could provide insight into expanding its use.
Conclusions
Our proposed GWNS scale is an AISS of LVO that is fast, feasible and highly predictive of LVO in the emergency department/hospital. Additionally, triaging and treating AIS with LVO using GWNS scale score, we may save golden time in AIS avoiding CTA and exposure to unnecessary radiation. The GWNS stroke scale outperformed the NIHSS in the detection of LVO despite NIHSS being the gold standard for comprehensive evaluation of a stroke patient, which must be performed after initial triage of LVO in the anterior circulation. The GWNS scale is not a substitution for NIHSS, but during an ultrafast triage of AIS with LVO, GWNS scale may help tremendously for early detection and perfusion therapy (Table 1). Further studies are needed to see if early perfusion therapy using GWNS scale score improves outcomes, which we are conduction now.
Scope of implication of the GWNS stroke scale of LVO in the developing countries like Bangladesh:
The major challenges in the developing countries are the poor infrastructure of an organized systemic care including non-mature acute stroke system care pathways. Additionally, the basic fundamentals of stroke evaluation using advanced imaginings are scars and not available in most of the places, and not affordable. Therefore, a pure clinical ischemic stroke scale of LVO like “GWNS” in the hospital and in acute care setting may significantly improve the stroke system care pathways by identifying severe disabling and life-threatening stroke promptly. The GWNS stroke scale LVO is simple to perform and takes only few minutes. More importantly, the GWNS scale is 4 points binary scale which makes it easy to learn, perform, implement and has highest correlation with LVO in the anterior circulation. Therefore, if the GWNS scale is 3 or higher, those patients could be sent directly for EVMT after a plain CT head with ASPECT, as the CT scanner are readily available to the developing countries including Bangladesh. As most of the regional hospitals in the developing countries have cardiac interventional programs, which could be used a Hybridge platform for EVMT for LVO in acute ischemic stroke. Now, we need to train all existing cardiac, vascular and peripheral endovascular specialists to create a mass of operators, who can lead the pathway. Author believe that a collaborative dialogue among all specialists with incite from private, local and internal organization is must for the future of stroke care in the developing countries like Bangladesh. The lead author Lodi et.al. strongly believes that ultimate power and the key of success lays in the hand of the supreme leader of a developing country, and their wisdom and willingness to care for their people.
References
1. Malhotra K, Gornbein J, Saver JL. Ischemic Strokes Due to Large-Vessel Occlusions Contribute Disproportionately to Stroke-Related Dependence and Death: A Review. Front Neurol. 2017;8:651. doi:10.3389/fneur.2017.00651
2. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. Jan 1 2015;372(1):11-20. doi:10.1056/NEJMoa1411587
3. Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. Mar 12 2015;372(11):1009-18. doi:10.1056/NEJMoa1414792
4. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. Mar 12 2015;372(11):1019-30. doi:10.1056/NEJMoa1414905
5. Molina CA, Chamorro A, Rovira A, et al. REVASCAT: a randomized trial of revascularization with SOLITAIRE FR device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int J Stroke. Jun 2015;10(4):619-26. doi:10.1111/ijs.12157
6. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. Feb 22 2018;378(8):708-718. doi:10.1056/NEJMoa1713973
7. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. Jan 4 2018;378(1):11-21. doi:10.1056/NEJMoa1706442
8. Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. Oct 2015;46(10):3020-35. doi:10.1161/STR.0000000000000074
9. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. Dec 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211
10. Campbell BCV, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol. Aug 2015;14(8):846-854. doi:10.1016/S1474-4422(15)00140-4
11. Saver JL, Goyal M, van der Lugt A, et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA. Sep 27 2016;316(12):1279-88. doi:10.1001/jama.2016.13647
12. Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology. Dec 12 2000;55(11):1649-55. doi:10.1212/wnl.55.11.1649
13. Lima FO, Silva GS, Furie KL, et al. Field Assessment Stroke Triage for Emergency Destination: A Simple and Accurate Prehospital Scale to Detect Large Vessel Occlusion Strokes. Stroke. Aug 2016;47(8):1997-2002. doi:10.1161/STROKEAHA.116.013301
14. Perez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. Jan 2014;45(1):87-91. doi:10.1161/STROKEAHA.113.003071
15. Tirschwell DL, Longstreth WT, Jr., Becker KJ, et al. Shortening the NIH Stroke scale for use in the prehospital setting. Stroke. Dec 2002;33(12):2801-6. doi:10.1161/01.str.0000044166.28481.bc
16. Hopyan JJ, Gladstone DJ, Mallia G, et al. Renal safety of CT angiography and perfusion imaging in the emergency evaluation of acute stroke. AJNR Am J Neuroradiol. Nov 2008;29(10):1826-30. doi:10.3174/ajnr.A1257
17. McCullough PA, Choi JP, Feghali GA, et al. Contrast-Induced Acute Kidney Injury. J Am Coll Cardiol. Sep 27 2016;68(13):1465-1473. doi:10.1016/j.jacc.2016.05.099
18. Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. Apr 2013;44(4):1153-7. doi:10.1161/STROKEAHA.111.000604
19. Kesinger MR, Sequeira DJ, Buffalini S, Guyette FX. Comparing National Institutes of Health Stroke Scale among a stroke team and helicopter emergency medical service providers. Stroke. Feb 2015;46(2):575-8. doi:10.1161/STROKEAHA.114.007850
20. Krebs W, Sharkey-Toppen TP, Cheek F, et al. Prehospital Stroke Assessment for Large Vessel Occlusions: A Systematic Review. Prehosp Emerg Care. Mar-Apr 2018;22(2):180-188. doi:10.1080/10903127.2017.1371263
21. Lodi Y, Reddy V, Petro G, Devasenapathy A, Hourani A, Chou CA. Primary acute stroke thrombectomy within 3 h for large artery occlusion (PAST3-LAO): a pilot study. J Neurointerv Surg. Apr 2017;9(4):352-356. doi:10.1136/neurintsurg-2015-012172
22. Yang P, Zhang Y, Zhang L, et al. Endovascular Thrombectomy with or without Intravenous Alteplase in Acute Stroke. N Engl J Med. May 21 2020;382(21):1981-1993. doi:10.1056/NEJMoa2001123
23. Suzuki K, Matsumaru Y, Takeuchi M, et al. Effect of Mechanical Thrombectomy Without vs With Intravenous Thrombolysis on Functional Outcome Among Patients With Acute Ischemic Stroke: The SKIP Randomized Clinical Trial. JAMA. Jan 19 2021;325(3):244-253. doi:10.1001/jama.2020.23522
24. Abbas R, Herial NA, Naamani KE, et al. Mechanical Thrombectomy in Patients Presenting with NIHSS Score <6: A Safety and Efficacy Analysis. J Stroke Cerebrovasc Dis. Mar 2022;31(3):106282. doi:10.1016/j.jstrokecerebrovasdis.2021.106282