Uncovering an Uncommon Outcome: A Case Report of Colchicine-Induced Aplastic Anemia
Ayesha Mohan B.S,Farzana Hoque M.D. MRCP (UK) FACP FRCP
Saint Louis University School of Medicine, St. Louis, MO, USA
Associate Professor of Medicine, Saint Louis University School of Medicine, St. Louis, MO, USA
Corresponding Authors: Ayesha Mohan B.S.
Correspondence: Saint Louis University School of Medicine, St. Louis, MO, USAAplastic anemia because of immunosuppressive drugs such as Colchicine is a rare consequence of a commonly prescribed treatment for various common diseases such as gout. It is crucial to have a high clinical suspicion of aplastic anemia when a patient who has recently started Colchicine presents with nonspecific symptoms such as fatigue, weakness, or recurrent infections. This is a unique case of a 68-year-old African American female who presented with persistent fatigue, generalized weakness, and dizziness upon standing that began approximately 3 months following the initiation of Colchicine for a new diagnosis of gout.
Keyword: drug-induced aplastic anemia bone marrow suppression aplastic anemia pancytopenia colchicine drug toxicity.
Uncovering an Uncommon Outcome: A Case Report of Colchicine-Induced Aplastic Anemia
Ayesha Mohan B.S,Farzana Hoque M.D. MRCP (UK) FACP FRCP
Saint Louis University School of Medicine, St. Louis, MO, USA
Associate Professor of Medicine, Saint Louis University School of Medicine, St. Louis, MO, USA
Corresponding Authors: Ayesha Mohan B.S.
Correspondence: Saint Louis University School of Medicine, St. Louis, MO, USA
Introduction
Colchicine is a well-established medication used to treat gout and various other immunological diseases. Due to its narrow therapeutic index and long half-life, colchicine toxicity is an important condition to be aware of due to its potential side effects. Acute colchicine poisoning can lead to GI disorders, shock, myelosuppression, and potentially death.1 Bone marrow aplasia is a frequent complication of colchicine poisoning and will characteristically occur on day 3 to 5 of initial drug exposure with continued suppression of blood cell counts for a week or longer.2 Most deaths linked to acute colchicine toxicity occur during the first week to 10 days following ingestion and can be attributed to early cardiorespiratory collapse or complications of delayed bone marrow suppression.2 Recognizing this rare but possible complication of a commonly used medication is essential for the timely management of a potentially life-threatening condition.
Case Report
We report a case of a 68-year-old African American female with a history of lupus on hydroxychloroquine and azathioprine, type II diabetes, hypertension, hyperlipidemia, chronic kidney disease, sleep apnea, and gout who presented to our facility with persistent and worsening fatigue, generalized weakness, and dizziness upon standing that began following the initiation of colchicine and allopurinol for her recent diagnosis of gout 2 months ago. Since beginning these medications, she has also noted bouts of non-bloody diarrhea, reduced appetite, and mouth sores.
During the week of admission, the patient had labs drawn in her rheumatologist’s office and they were significant for pancytopenia, WBC of 1×109/L, hemoglobin (Hb) of 6.4 g/dL & platelet 31 × 109/L. She was sent to the emergency department (ED) and underwent 1 unit packed red blood cell transfusion. Physical exam in the ED notable for mucosal breakdown in the mouth with white plaque noted on the left side of her tongue. Workup to investigate alternative causes for pancytopenia included direct antiglobulin test, HIV, Hepatitis B, Hepatitis C, EBV PCR, vitamin B12, folate, and copper were unremarkable. Inflammatory markers ESR and C-reactive protein were elevated at 53 mm/hr and 5.1 mg/L respectively with no hemolysis noted. We discontinued colchicine and advised celecoxib 200 mg for joint pain and swelling. The CT chest, abdomen, and pelvis did not show hepatosplenomegaly or lymphadenopathy. A bone marrow biopsy showed markedly hypocellular marrow with virtually absent myelo and erythropoiesis, resembling aplastic anemia. We also ordered flow cytometry, and no clonal B-cell or increased blast formation was detected indicating a lack of evidence of lymphoma or high-grade myeloid neoplasm. Further workup included chromosomal analysis, FISH CML/ALL, and myeloid malignancy panel all of which were within normal limits. Flow cytometry showed no peripheral clonal B-cell or aberrant T-cell population and was negative for paroxysmal nocturnal hemoglobinuria. Reduced reticulocyte count supported myelosuppression. Colchicine and allopurinol were held for the entirety of her admission and upon discharge. Approximately a month following discharge, she was seen in the Hematology outpatient clinic where her energy levels had improved and she denied any bleeding, fevers, chills, shortness of breath, chest pain, or diarrhea. At this time, she continued to experience gout pain in her bilateral lower extremities and states her pain has not been controlled since discharge. Since stopping colchicine in the hospital, the patient’s blood counts began to recover. The extensive workup for alternative causes for pancytopenia was negative leading to the conclusion that colchicine was the reason for the pancytopenia. The patient has not had further signs or symptoms of pancytopenia since this admission to our knowledge.
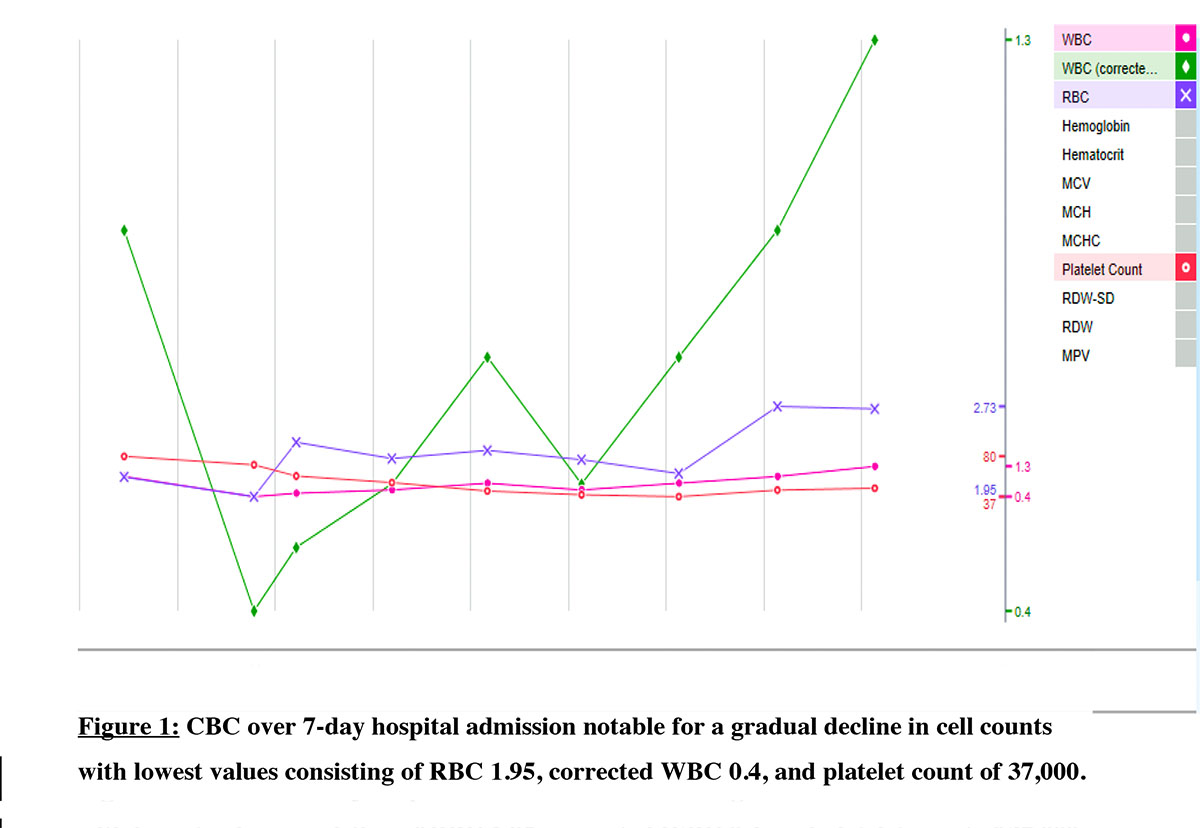
Discussion
Drug-induced aplastic anemia is a potentially life-threatening reaction associated with certain pharmacological therapies and can lead to bone marrow toxicity and subsequent pancytopenia. Early signs of this syndrome include fatigue, frequent infections, persistent bleeding, weakness, dizziness, and headache.3 There are currently no established guidelines to prevent aplastic anemia although avoiding exposure to insecticides, herbicides, organic solvents, and other toxic chemicals could potentially lower the risk of development.4 Many different medications including chloramphenicol, phenylbutazone, sulfonamides, anticonvulsants, cimetidine, and others have the potential to cause aplastic anemia.5 The incidence of diagnosis varies by region, ranging from 1.5-7 cases per million people yearly, and the median age at diagnosis is 25-60 years.6 The two proposed mechanisms for drug-induced aplastic anemia include dose-dependent reversible toxicity and idiosyncratic toxicity with high mortality.7 Studies have shown that younger aplastic anemia patients experience an overall optimistic long-term survival while patients greater than 60 years of age remain to have a poorer prognosis.6 Chloramphenicol is most commonly linked to aplastic anemia with the estimated incidence being 1 in 20,000 among patients treated with chloramphenicol.7 Colchicine has been advised to be used with caution in people with prior renal or hepatic impairment and those in the older age group due to the potential for iatrogenic toxicity leading to bone marrow suppression. A retrospective study by Singh, et al. showed that a diagnosis of gout itself increases the risk of experiencing aplastic anemia and pancytopenia.8 The use of colchicine, regardless of the setting of gout, increases the risk of anemia by 3 to 4-fold and 2 to 3-fold for pancytopenia.8 Colchicine may induce aplastic anemia a few days following exposure (3 to 5 days) and may resolve a few days to weeks following cessation. Nausea, vomiting, and diarrhea are common symptoms of presentation of acute toxicity. 9 Colchicine-induced pancytopenia patients are at risk for life-threatening serious conditions. Treatment with granulocyte-colony stimulating factor (G-CSF) is sometimes given to increase the low counts, although recent studies have demonstrated that there is no evidence that G-CSF can correct the underlying hematopoietic stem/progenitor cell defect in aplastic anemia. It has also been shown that G-CSF does not alter the course of the disease, improve response to immunosuppressive therapy, or increase overall survival.10 Reviewing a patient’s medication list when noticing the signs and symptoms of pancytopenia is imperative. Deprescribing potential drugs such as colchicine for our patient immediately upon recognition of its potential bone marrow suppression is important to ensure minimal long-term effects of this drug-induced complication.
Conclusion
Colchicine-induced aplastic anemia in the setting of gout and other conditions has the potential to lead to fatal outcomes because of the implications of pancytopenia if not met with prompt diagnosis and definitive management. A multidisciplinary approach with early hematology intervention is vital to ensure safe patient outcomes. Clinicians’ awareness of this rare but highly fatal complication is imperative to prevent devastating outcomes.
- Wu J, Liu Z. Progress in the management of acute colchicine poisoning in adults. Intern Emerg Med. 2022;17(7):2069-2081. doi:10.1007/s11739-022-03079-6
- Roger Harris, Gavin Marx, Mark Gillett, Adrian Kark, Shalini Arunanthy.
Colchicine-induced bone marrow suppression: treatment with granulocyte colony-stimulating factor11. The Journal of Emergency Medicine, Vol. 18, Issue 4, 2000, Pages 435-440, ISSN 0736-4679. doi: 10.1016/S0736-4679(00)00160-8.
- Symptoms & Causes of Aplastic Anemia & Myelodysplastic Syndromes | NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases. Published July 2020. https://www.niddk.nih.gov/health-information/blood-diseases/aplastic-anemia-myelodysplastic-syndromes/symptoms-causes
- Mayo Clinic Staff. Aplastic anemia - Symptoms and causes. Mayo Clinic. Published February 11, 2022. https://www.mayoclinic.org/diseases-conditions/aplastic-anemia/symptoms-causes/syc-20355015
- Young NS. Acquired aplastic anemia. Ann Intern Med. 2002;136(7):534-546. doi:10.7326/0003-4819-136-7-200204020-00011
- Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000-2011. Haematologica. 2017;102(10):1683-1690. doi:10.3324/haematol.2017.169862
- Syed MA, Atta Ur Rahman A, Shah Syed MN, Memon NM. The Relationship of Drug Therapy to Aplastic Anemia in Pakistan: A Hospital-Based Case-Control Study. Ther Clin Risk Manag. 2021;17:903-908. Published 2021 Aug 27. doi:10.2147/TCRM.S325742
- Singh JA, Yang S, Foster J (2014) The risk of aplastic anemia and pancytopenia with colchicine: A Retrospective study of integrated health system database. Am College Rheumatol 66: S20-S20.
- Mingjie F, Jie Z, Zhitao L, He Z, Anwei L (2019) Clinical outcomes after colchicine overdose - A case report. Medicine 98: e16580.
- Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117(17):4434-4441. doi:10.1182/blood-2010-08-304071