Guidelines updates on heart failure management, a comprehensive and updated review
Muhammad Ghallab,Muhammad Haseeb ul Rasool,Zakaria Alagha,Reza Tahmid,Mahmoud Nassar,Hazem Abosheaishaa,Saad Javed, Most Munira
- Department of Cardiology, Assistant Professor of Clinical Medicine. Icahn School of Medicine at Mount Sinai |NYC Health + Hospitals Queens, New York, USA
- Department of internal medicine, Icahn School of Medicine at Mount Sinai |NYC Health + Hospitals Queens, New York, USA
- Department of internal medicine, Marshall University, Joan Edward, School of Medicine, West Virginia, USA
- Third year, Medicine MBChB, University of Glasgow, UK
Emails
- Muhammad Ghallab: m.ghallab91@gmail.com
- Muhammad Haseeb ul Rasool: haseeb219@gmail.com
- Zakaria Alagha: Zakariaalagh@marshall.edu
- Reza Tahnid: rezatahmid@gmail.com
- Mahmoud Nassar: dr.nassar@aucegypt.edu
- Hazem Abosheaishaa: hazemabosheaishaa@gmail.com
- Saad Javed: Javeds@nychhc.org
- Most Sirajum Munira: muniram1@nychhc.org
Corresponding author
Name: Muhammad Ghallab
Email: m.ghallab91@gmail.com
Affiliation: Department of internal medicine, Icahn School of Medicine at Mount Sinai |NYC Health + Hospitals Queens, New York, USA
Corresponding Authors: Muhammad Ghallab
Correspondence: Department of internal medicine, Icahn School of Medicine at Mount Sinai |NYC Health + Hospitals Queens, New York, USAAlmost 300,000 people die each year of heart failure (HF). The incidence of HF in the United States is 2.4%, and its prevalence is expected to jump by 46% by 2030; at that time, 1 in 33 Americans will have HF. This will impose a higher financial and health burden. Thus, the management of HF has evolved significantly over the past few decades with the introduction of different pharmacological agents that improve mortality and reduce hospitalization. The American College of Cardiology/American Heart Association/Heart Failure Society of America (ACC/AHA/HFSA) updated the HF guidelines for 2022 based on the available evidence over 40 years to mitigate that burden and improve survival. This state-of-the-art review aims to highlight the latest evidence regarding the pharmacological management of HF among all categories of HF based on ejection fraction. It focuses on the four medication pillars in HF and other beneficial medications. It emphasizes the recommended doses to achieve the maximal mortality benefit. The medications with some or no benefits or those with harmful effects in HF are also covered in this review.
Keyword: Congestive heart failure CHF Heart failure management Cardiovascular Cardiomyopathies
Guidelines updates on heart failure management, a comprehensive and updated review
Muhammad Ghallab,Muhammad Haseeb ul Rasool,Zakaria Alagha,Reza Tahmid,Mahmoud Nassar,Hazem Abosheaishaa,Saad Javed, Most Munira
- Department of Cardiology, Assistant Professor of Clinical Medicine. Icahn School of Medicine at Mount Sinai |NYC Health + Hospitals Queens, New York, USA
- Department of internal medicine, Icahn School of Medicine at Mount Sinai |NYC Health + Hospitals Queens, New York, USA
- Department of internal medicine, Marshall University, Joan Edward, School of Medicine, West Virginia, USA
- Third year, Medicine MBChB, University of Glasgow, UK
Emails
- Muhammad Ghallab: m.ghallab91@gmail.com
- Muhammad Haseeb ul Rasool: haseeb219@gmail.com
- Zakaria Alagha: Zakariaalagh@marshall.edu
- Reza Tahnid: rezatahmid@gmail.com
- Mahmoud Nassar: dr.nassar@aucegypt.edu
- Hazem Abosheaishaa: hazemabosheaishaa@gmail.com
- Saad Javed: Javeds@nychhc.org
- Most Sirajum Munira: muniram1@nychhc.org
Corresponding author
Name: Muhammad Ghallab
Email: m.ghallab91@gmail.com
Affiliation: Department of internal medicine, Icahn School of Medicine at Mount Sinai |NYC Health + Hospitals Queens, New York, USA
Corresponding Authors: Muhammad Ghallab
Correspondence: Department of internal medicine, Icahn School of Medicine at Mount Sinai |NYC Health + Hospitals Queens, New York, USA
Introduction
HF remains a significant concern, leading to substantial morbidity, hospitalizations, and mortality, which imposes a significant financial burden on healthcare systems worldwide. The management costs for HF can account for up to 2% of the total healthcare budget [1]. For over a century, the New York Heart Association (NYHA) functional classification has served as a gold standard for quantifying the health status of HF patients. However, it is essential to note that the NYHA score is susceptible to significant patient-reported bias, despite being the most used functional classification [2, 3]. To mitigate interpersonal bias, the American Heart Association has developed an objective-based functional classification, which allows for the independent classification of disease severity based on objective evidence, such as echocardiogram and cardiac catheterization results [3].
Treatment for HF encompasses four areas: lifestyle changes, medications, interventional devices, and surgery. Medical management involves Guideline-Directed Medical Therapy (GDMT), which has been used recently to maximize the survival benefit for HF patients. It has been demonstrated that GDMT dramatically reduces the clinical outcomes of HF with reduced ejection fraction. GDMT, or pharmacological intervention, relies on four pharmacological classes: Angiotensin Receptor-Neprilysin Inhibitor (ARNi)/Angiotensin receptor blockers (ARBs)/Angiotensin-converting enzyme inhibitors (ACEi), beta-blockers, mineralocorticoid Receptor Antagonist (MRA)/diuretics, and emerging Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors SGLT2 inhibitors [4].
The AHA and the ACC have provided clinical guidelines based on available clinical data since 1980. The latest guidelines, including updates on HF treatment, are jointly provided by the AHA, ACC, and HFSA. These guidelines build upon the previous recommendations made in the 2013 ACCF/AHA guidelines for managing HF in adults and the 2017 ACC/AHA/HFSA-focused update of the 2013 ACCF/AHA guidelines for managing HF [5, 6].
The updated guidelines from 2022 offer new recommendations regarding the use of SGLT2 inhibitors, ARNi, the management of HF in patients with Atrial fibrillation, and the management of secondary mitral regurgitation (MR) in HF, including mitral valve transcatheter edge-to-edge repair. Additionally, the guidelines provide updated recommendations on cardiac amyloidosis, cardiac oncology, implantable devices, and the utilization of left ventricular assist devices (LVADs) in the stage D HF [7].
2022 Guidelines on HF
HF is a term used to describe the physiological states in which the cardiac output is insufficient to meet the body's needs. It can result from structural or functional issues impairing the heart's ability to supply sufficient blood [8].
The diagnosis of HF requires a high clinical suspicion based on the patient's clinical history, examination findings, electrocardiogram (EKG), and laboratory work. B-type natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels should be measured to confirm elevated filling pressure.
Following laboratory tests, a transthoracic Echocardiogram should be performed to determine the ejection fraction, which helps classify the HF based on the severity of the reduction in ejection fraction. HF is now classified into four categories based on the ejection fraction: HFrEF (heart failure with reduced ejection fraction), HFmrEF (heart failure with mildly reduced ejection fraction), HFpEF (heart failure with preserved ejection fraction), and HEimpEF (heart failure with improved ejection fraction). The corresponding ejection fraction ranges for these categories are as follows: HFrEF <40%, HFmrEF 40-49%, HEpEF >50%, and HEimpEF with improvement in ejection fraction (HFpEF) [9, 10]. All patients with current or prior HF, irrespective of EF, should be considered for GDMT.
Please note that the new guideline includes the addition of the HFimpEF category, which represents heart failure with improved ejection fraction. Additionally, according to the European Society of Cardiology (ESC), HFmrEF is classified as a mid-range ejection fraction, while the AHA classifies it as a mildly reduced ejection fraction.
The recommendations for treatment have been quantified into the following classes [11]
- Class 1 (Strong: Benefit>>> Risk)
- Class 2a (Moderate: Benefit>> Risk)
- Class 2b (Weak: Benefit ≥Risk)
- Class 3: No Benefit (Moderate)
- Class 3: Harm (Strong)
The quality or level of evidence to support the guidelines is graded as follows:
- Level A: High-quality evidence based on more than one randomized controlled trial (RCT), a meta-analysis of high-quality RCTs, or one or more RCTs supported by high-quality registry studies.
- Level B-R (Randomized): Moderate-quality evidence based on one or more RCTs or a meta-analysis of moderate-quality RCTs.
- Level B-NR (Non-Randomized): Moderate-quality evidence from one or more well-designed and well-executed non-randomized studies, observational studies, or registry studies, or from a meta-analysis of these studies.
- Level C-LD (Limited Data): Evidence from randomized or non-randomized observational or registry studies with limitations in design or execution, a meta-analysis of such studies, or evidence based on physiological or mechanistic studies in human subjects.
- Level C-EO (Expert Opinion): Consensus of expert opinion based on clinical experience.
Guideline-directed medical therapy (GDMT): The review will primarily focus on GDMT
Renin-Angiotensin-Aldosterone System Inhibition (RAASi) with ACEi, ARBs, or ARNi
ARNi, ARBs, and ACEi are the first pillar of pharmacological management in HFrEF. RAAS inhibition is supported by a Class 1 recommendation and Level A evidence for patients with HFrEF and NYHA class II and III symptoms. The use of ARNi in patients with HFrEF experiencing NYHA Class II and III symptoms is associated with a significant reduction in morbidity and mortality. In cases where ARNi use is not feasible, ACEi should be used. ARB use is recommended to reduce morbidity and mortality if the patient cannot tolerate ACEi due to cough or angioedema. The use of ARBs and ACEi provides high economic value. Patients with HFrEF and Class II or III NYHA symptoms who can tolerate ACEi or ARB should be switched to ARNi to reduce morbidity and mortality further. It is recommended that ARNi should not be administered concomitantly with ACEi or at least within 36 hours of the last dose of an ACEi. ARNi should also be avoided for patients with a history of angioedema. [12-14]
Beta-blockers
Carvedilol, metoprolol succinate, and bisoprolol are recommended for HFrEF, and maximum mortality benefit is observed when the dose is titrated to the maximum tolerable dose [15].
Mineralocorticoid Receptor Antagonist (MRAs)
Mineralocorticoid receptor antagonists (MRAs) are the third pillar of GDMT for HF with reduced ejection fraction (HFrEF). They are supported by a Class 1 recommendation and Level A evidence for patients with HFrEF and NYHA class II and III symptoms. It is recommended to initiate MRAs, such as spironolactone and eplerenone, if the estimated glomerular filtration rate (eGFR) is >30 mL/min/1.73m² and serum potassium is <5.0 mEq/L. The use of MRAs requires careful monitoring of potassium levels and renal function. Diuretic doses should be adjusted at initiation, and close monitoring is necessary to detect hyperkalemia and renal insufficiency. If patients have difficulty maintaining serum potassium levels below 5.5 mEq/L, MRAs should be discontinued to prevent life-threatening hyperkalemia [16, 17].
SGLT2 Inhibitors
SGLT2 inhibitors are the 4th pillar of treatment for HFrEF. Dapagliflozin has been approved first in the USA for reducing the risk of cardiovascular death and hospitalization for HF in adults with NYHA class II-IV HFrEF, whether they have type 2 diabetes or not. Canagliflozin and empagliflozin are other SGLT2 inhibitors approved in this class [18-20].
Hydralazine and Isosorbide Dinitrate
Hydralazine and isosorbide dinitrate carry level 1 recommendation and Class A level of evidence for patients identified as African American who have HFrEF with NYHA class II–IV symptoms and are already on optimized medical therapy. Hydralazine and isosorbide dinitrate have been shown to improve symptoms and reduce mortality in the specific group. Hydralazine and isosorbide dinitrate have also been recommended for patients who cannot be on RAASi medications because of drug intolerance or renal insufficiency to reduce mortality and morbidity [21, 22].
Medications with no mortality benefits
- Ivabradine has been used in patients who are on the maximum beta-blocker dose and have a sinus rate of >70 bpm or cannot tolerate beta-blockers. No mortality benefits have been demonstrated (Class IIa recommendation) [23].
- Digoxin provides no mortality benefit and can even be harmful in scenarios of electrolyte abnormalities. It is recommended only in cases of CHF with atrial fibrillation [24].
- Diuretics only provide symptomatic control with no mortality benefit. For patients admitted with HF exacerbations, diuretics are always given via IV as gut edema reduces the absorption of diuretics [25].
- Soluble Guanylyl Cyclase Stimulators: Oral Soluble guanylyl cyclase stimulators include vericiguat, which binds directly and stimulates soluble guanylyl cyclase and increases cyclic guanosine monophosphate (cGMP) production leading to vasodilation, remodeling in endothelial function, and decreased cardiac fibrosis and remodeling. It is recommended for high-risk patients with progressive worsening LVEF despite being on GDMT for HFrEF to reduce heart failure hospitalization and cardiovascular deaths [26].
Medications with some benefits
- Patients who are on RAASi medications and are experiencing hyperkalemia (Potassium >5.5mEq/L) can be prescribed Potassium Binders (patiromer, Sodium Zirconium Cyclosilicate). However, the benefit is unclear [27].
- Omega-3 polyunsaturated fatty acids provide an adjunctive therapy to reduce mortality and cardiovascular hospitalization [28]. This treatment approach is classified as Class IIb.
Medications with no benefits (Class III: Moderate):
- Anticoagulation is not recommended in HFrEF without another clinical indication, including VTE, PE, AF, or cardiogenic embolic source [29, 30].
- Dihydropyridine calcium channel-blocking drugs, including nifedipine and amlodipine, are not recommended in HFrEF [31].
- Nutritional supplements, including vitamins and hormonal therapy, are not recommended [32-34]
Medications with harmful effect[MSM1] s (Class III: Strong) Contraindicated:
- Non-Dihydropyridine calcium channel-blocking drugs, such as diltiazem and verapamil, are not recommended in HFrEF [35, 36].
- Class 1C antiarrhythmic medication and dronedarone may increase the risk of mortality [37].
- Thiazolidinediones, including rosiglitazone and pioglitazone, may worsen HF symptoms and increase hospitalization if used for type 2 diabetes [38].
- Dipeptidyl peptidase-4 (DPP-4) inhibitors, including saxagliptin and alogliptin, increase hospitalization risk when used for type 2 diabetes treatment [39, 40].
- NSAIDs may worsen HF and should be avoided as much as possible [41].
HFimpEF
HFimpEF is a subgroup of patients with HFrEF who experience improved left ventricular ejection fraction (LVEF) once they start GDMT. It is recommended that GDMT should be continued to prevent the relapse of HF and left ventricular (LV) dysfunction, even if the patient is asymptomatic [42].
HFmrEF
Patients with HF and LVEF between 41%-49% should be started on diuretics and SGLT2 inhibitors. The use of MRA, RAASi, and beta-blockers has a 2b level of recommendation [43].
GDMT dosing: sequencing and up-titration
HF treatment aims to maximize the use of medications to achieve the maximum recommended dose, supported by randomized controlled trials (RCTs), to obtain the most significant mortality benefit. It is recommended to titrate and optimize GDMT as frequently as every 1-2 weeks, considering patient symptoms, vital signs, and laboratory findings.
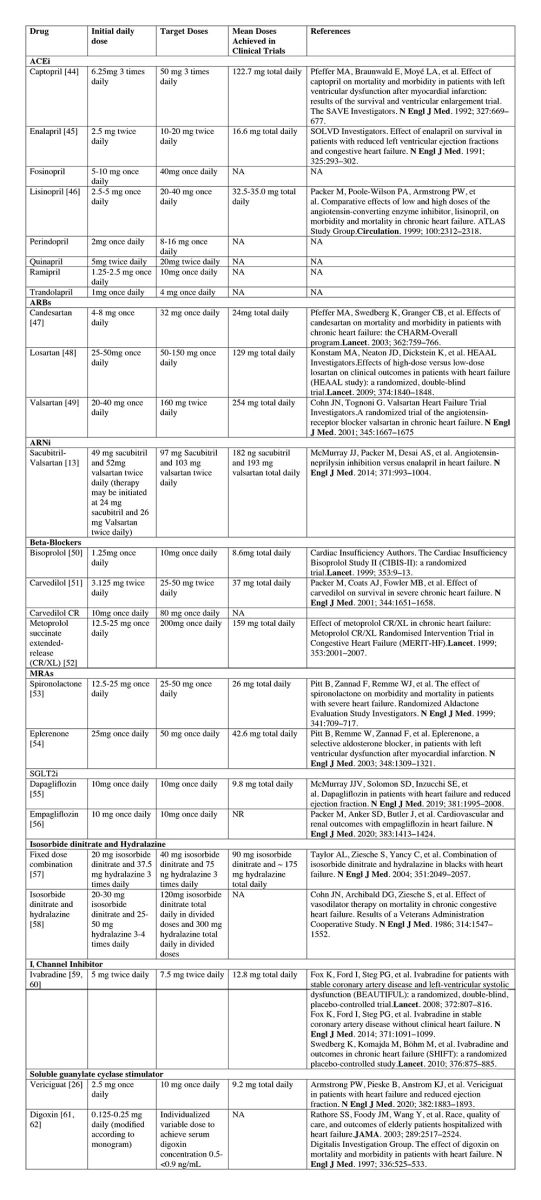
HFpEF
Patients with HFpEF and HTN should have medications titrated following published guidelines to achieve the target blood pressure. In patients with HFpEF, SGLT2 inhibitors can be beneficial in reducing HF hospitalizations and cardiovascular mortality. Managing AF in HFpEF patients can help improve symptoms. In selected patients with HFpEF, MRA may be considered to decrease hospitalizations, especially among patients with LVEF. ARBs and ARNi can be considered in HFpEF patients with LVEF on the lower end of the spectrum. However, the routine use of nitrates or phosphodiesterase-5 inhibitors to increase activity or improve quality of life (QOL) is ineffective in patients with HFpEF [43, 63, 64].
Conclusion
HF is the leading cause of morbidity and mortality in both developing and developed countries, with the cost of management accounting for approximately 2% of global health expenses. The AHA and ACC have provided updated guidelines on managing HF since 1980. GDMT has become the gold standard for medical therapy in HF. It includes using RAASi, beta-blockers, MRAs, and SGLT2 inhibitors. It is essential to titrate each class of medication to the maximally tolerated doses for the patient to achieve the maximum mortality benefit. For patients who show improvement in LVEF, it is necessary to continue GDMT to prevent relapse of HF.
1. Chang, H.Y., et al., Gap between guidelines and clinical practice in heart failure with reduced ejection fraction: Results from TSOC-HFrEF registry. J Chin Med Assoc, 2017. 80(12): p. 750-757.
2. Greene, S.J., et al., Comparison of New York Heart Association Class and Patient-Reported Outcomes for Heart Failure With Reduced Ejection Fraction. JAMA Cardiol, 2021. 6(5): p. 522-531.
3. Gidding, S.S., et al., Prevalence of American Heart Association Heart Failure Stages in Black and White Young and Middle-Aged Adults: The CARDIA Study. Circ Heart Fail, 2019. 12(9): p. e005730.
4. Haydock, P.M. and A.S. Flett, Management of heart failure with reduced ejection fraction. Heart, 2022. 108(19): p. 1571-1579.
5. Yancy, C.W., et al., 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 2013. 62(16): p. e147-239.
6. Yancy, C.W., et al., 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation, 2017. 136(6): p. e137-e161.
7. Heidenreich, P.A., et al., 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation, 2022. 145(18): p. e895-e1032.
8. Ammar, K.A., et al., Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation, 2007. 115(12): p. 1563-70.
9. Madsen, B.K., et al., Chronic congestive heart failure. Description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J, 1994. 15(3): p. 303-10.
10. Ponikowski, P., et al., 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J, 2016. 37(27): p. 2129-2200.
11. Foundation, A.C.o.C., Methodology manual and policies from the ACCF/AHA Task Force on Practice Guidelines. http://my. americanheart. org/idc/groups/ahamah-public/@ wcm/@ sop/documents/downloadable/ucm_319826. pdf, 2010.
12. Wang, Y., et al., Effects of the Angiotensin-Receptor Neprilysin Inhibitor on Cardiac Reverse Remodeling: Meta-Analysis. J Am Heart Assoc, 2019. 8(13): p. e012272.
13. McMurray, J.J., et al., Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med, 2014. 371(11): p. 993-1004.
14. Yusuf, S., et al., Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet, 2008. 372(9644): p. 1174-83.
15. Cleland, J.G.F., et al., Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J, 2018. 39(1): p. 26-35.
16. Glick, H.A., et al., Economic evaluation of the randomized aldactone evaluation study (RALES): treatment of patients with severe heart failure. Cardiovasc Drugs Ther, 2002. 16(1): p. 53-9.
17. Zhang, Z., et al., Cost effectiveness of eplerenone in patients with heart failure after acute myocardial infarction who were taking both ACE inhibitors and beta-blockers: subanalysis of the EPHESUS. Am J Cardiovasc Drugs, 2010. 10(1): p. 55-63.
18. Isaza, N., et al., Cost-effectiveness of Dapagliflozin for the Treatment of Heart Failure With Reduced Ejection Fraction. JAMA Netw Open, 2021. 4(7): p. e2114501.
19. Neal, B., V. Perkovic, and D.R. Matthews, Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med, 2017. 377(21): p. 2099.
20. Zannad, F., et al., SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet, 2020. 396(10254): p. 819-829.
21. Khazanie, P., et al., Clinical Effectiveness of Hydralazine-Isosorbide Dinitrate Therapy in Patients With Heart Failure and Reduced Ejection Fraction: Findings From the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail, 2016. 9(2): p. e002444.
22. Angus, D.C., et al., Cost-effectiveness of fixed-dose combination of isosorbide dinitrate and hydralazine therapy for blacks with heart failure. Circulation, 2005. 112(24): p. 3745-53.
23. Swedberg, K., et al., Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet, 2010. 376(9744): p. 875-85.
24. Malik, A., et al., Digoxin Discontinuation and Outcomes in Patients With Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol, 2019. 74(5): p. 617-627.
25. Murray, M.D., et al., Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med, 2001. 111(7): p. 513-20.
26. Armstrong, P.W., et al., Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med, 2020. 382(20): p. 1883-1893.
27. Pitt, B., et al., Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J, 2011. 32(7): p. 820-8.
28. Tavazzi, L., et al., Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet, 2008. 372(9645): p. 1223-30.
29. Homma, S., et al., Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med, 2012. 366(20): p. 1859-69.
30. Zannad, F., et al., Rivaroxaban in Patients with Heart Failure, Sinus Rhythm, and Coronary Disease. N Engl J Med, 2018. 379(14): p. 1332-1342.
31. Packer, M., et al., Effect of amlodipine on the survival of patients with severe chronic heart failure due to a nonischemic cardiomyopathy: results of the PRAISE-2 study (prospective randomized amlodipine survival evaluation 2). JACC Heart Fail, 2013. 1(4): p. 308-314.
32. Djoussé, L., et al., Supplementation With Vitamin D and Omega-3 Fatty Acids and Incidence of Heart Failure Hospitalization: VITAL-Heart Failure. Circulation, 2020. 141(9): p. 784-786.
33. Marchioli, R., et al., Vitamin E increases the risk of developing heart failure after myocardial infarction: Results from the GISSI-Prevenzione trial. J Cardiovasc Med (Hagerstown), 2006. 7(5): p. 347-50.
34. Mortensen, S.A., et al., The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail, 2014. 2(6): p. 641-9.
35. The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med, 1988. 319(7): p. 385-92.
36. Goldstein, R.E., et al., Diltiazem increases late-onset congestive heart failure in postinfarction patients with early reduction in ejection fraction. The Adverse Experience Committee; and the Multicenter Diltiazem Postinfarction Research Group. Circulation, 1991. 83(1): p. 52-60.
37. Echt, D.S., et al., Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med, 1991. 324(12): p. 781-8.
38. Lago, R.M., P.P. Singh, and R.W. Nesto, Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet, 2007. 370(9593): p. 1129-36.
39. Home, P.D., et al., Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet, 2009. 373(9681): p. 2125-35.
40. Scirica, B.M., et al., Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation, 2014. 130(18): p. 1579-88.
41. Gislason, G.H., et al., Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med, 2009. 169(2): p. 141-9.
42. Halliday, B.P., et al., Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet, 2019. 393(10166): p. 61-73.
43. Thorvaldsen, T., et al., Triage of patients with moderate to severe heart failure: who should be referred to a heart failure center? J Am Coll Cardiol, 2014. 63(7): p. 661-671.
44. Pfeffer, M.A., et al., Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med, 1992. 327(10): p. 669-77.
45. Yusuf, S., et al., Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med, 1991. 325(5): p. 293-302.
46. Packer, M., et al., Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation, 1999. 100(23): p. 2312-8.
47. Pfeffer, M.A., et al., Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet, 2003. 362(9386): p. 759-66.
48. Konstam, M.A., et al., Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet, 2009. 374(9704): p. 1840-8.
49. Cohn, J.N. and G. Tognoni, A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med, 2001. 345(23): p. 1667-75.
50. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet, 1999. 353(9146): p. 9-13.
51. Packer, M., et al., Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med, 2001. 344(22): p. 1651-8.
52. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet, 1999. 353(9169): p. 2001-7.
53. Pitt, B., et al., The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med, 1999. 341(10): p. 709-17.
54. Pitt, B., et al., Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med, 2003. 348(14): p. 1309-21.
55. McMurray, J.J.V., et al., Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med, 2019. 381(21): p. 1995-2008.
56. Packer, M., et al., Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med, 2020. 383(15): p. 1413-1424.
57. Taylor, A.L., et al., Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med, 2004. 351(20): p. 2049-57.
58. Cohn, J.N., et al., Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med, 1986. 314(24): p. 1547-52.
59. Fox, K., et al., Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet, 2008. 372(9641): p. 807-16.
60. Fox, K., et al., Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med, 2014. 371(12): p. 1091-9.
61. Rathore, S.S., et al., Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. Jama, 2003. 289(19): p. 2517-24.
62. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med, 1997. 336(8): p. 525-33.
63. Anker, S.D., et al., Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. Eur J Heart Fail, 2019. 21(10): p. 1279-1287.
64. Kalogirou, F., et al., Heart failure disease management: a systematic review of effectiveness in heart failure with preserved ejection fraction. ESC Heart Fail, 2020. 7(1): p. 194-212.