Oncologic Emergencies: Presentations and Principles of Management
Vinh Dao MD,Asit K. Paul MD. PhD
1. Virginia Commonwealth University School of Medicine, Richmond, VA
2. Division of Hematology, Oncology and Palliative Care, Virginia Commonwealth University, Richmond, VA
2. Massey Cancer Center, Virginia Commonwealth University, Richmond, VA
Corresponding Authors: Asit K. Paul MD. PhD
Correspondence: Virginia Commonwealth University School of Medicine, Richmond, VAPatients with cancers often present to emergency department with an acute onset of symptoms, so-called oncologic emergencies. An oncologic emergency may arise as an initial presentation, a consequence of cancer progression or a complication of therapy. Most oncologic emergencies require interdisciplinary management. In this review, we discuss some common oncologic emergencies and principles of their management.
Oncologic Emergencies: Presentations and Principles of Management
Vinh Dao MD,Asit K. Paul MD. PhD
1. Virginia Commonwealth University School of Medicine, Richmond, VA
2. Division of Hematology, Oncology and Palliative Care, Virginia Commonwealth University, Richmond, VA
2. Massey Cancer Center, Virginia Commonwealth University, Richmond, VA
Corresponding Authors: Asit K. Paul MD. PhD
Correspondence: Virginia Commonwealth University School of Medicine, Richmond, VA
Introduction
The American Cancer Society estimates 1,958,310 new cancer cases and 609,820 cancer related deaths in 2023.1 Despite advances in diagnostics and therapies, cancer patients die from complications related to cancer and therapies. Each year there are approximately 4 million visits to the emergency department due to their cancer-related symptoms.2 About 11-29% patients are diagnosed with cancers after an emergency room visit.2 Oncologic emergencies vary in terms of their presentation with some being insidious at the beginning while others manifesting within hours with the potential to cause serious life-threatening complications, such as sepsis, respiratory distress, cardiac arrythmias, renal failure, electrolyte imbalances and death. Patients with a history of cancer often lack the classic red-flag symptoms associated with emergent conditions. Physicians should have a low suspicion for completing a thorough workup so that prompt treatment can be implemented without delay. Many of these emergent conditions (Table 1) could be managed through interdisciplinary expertise with a positive outcome if intervened early.
Table 1. Common Oncologic Emergencies
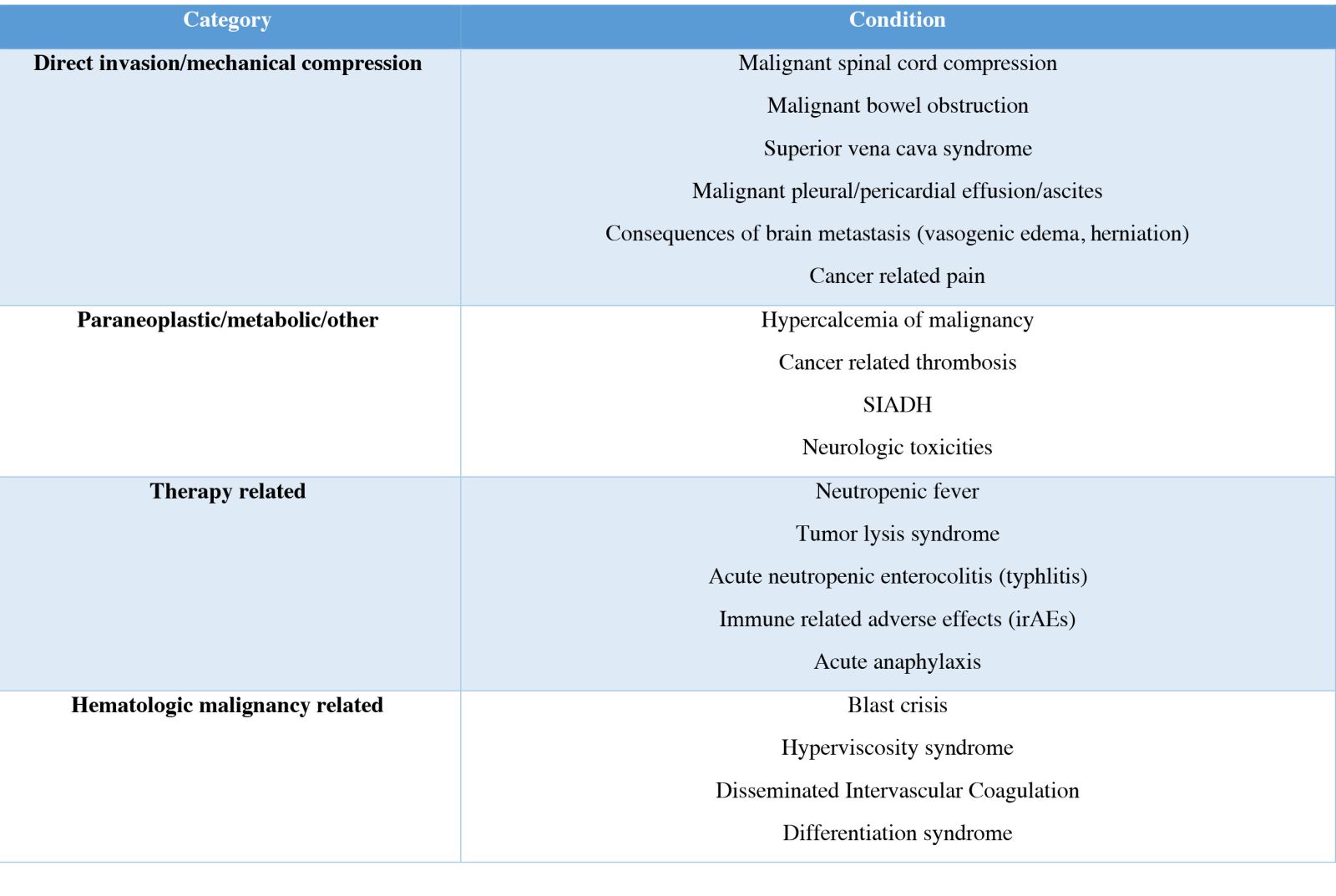
In this review, we discuss some selected and common oncologic emergencies and their management.
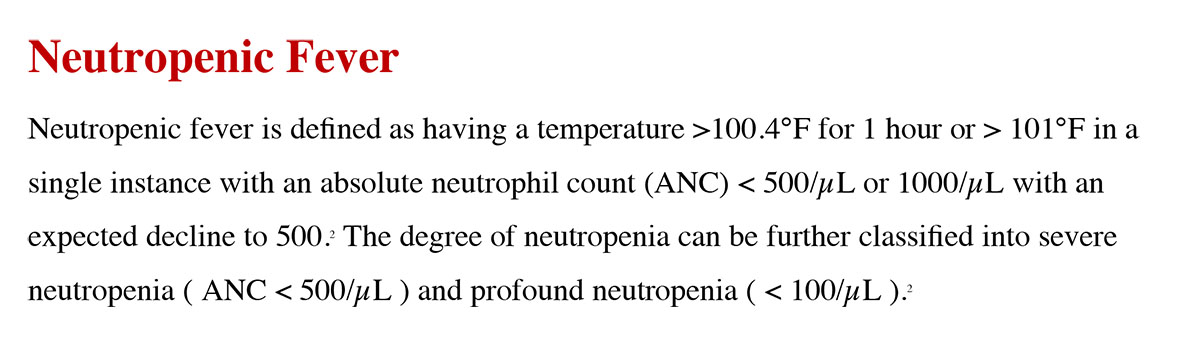
The ANC can be decreased due to cytotoxic effects of chemotherapy or due to the particular type of cancer exhibited, typically leukemia or metastatic cancers that affects the bone marrow.3 Although one of the most common causes of febrile neutropenia is bacterial infection, fungal infections, particularly in patients with chronic neutropenia and hematologic cancer, are also often seen.4
Neutropenic fevers are often difficult to treat as the signs and symptoms associated with them can be subtle and often missed. As such, knowing several risk factors that can increase the likelihood of having febrile neutropenia can be of great value for physicians. Several algorithms have been found to identify patients at risk for complications from neutropenic fever, in particular the Multinational Association for Supportive Care in Cancer risk (MASCC) index.5 Common sources include respiratory tract, gastrointestinal tract, urinary tract and vascular catheter. Even with extensive work-up, a source of infection may not always be found. Treatment for neutropenic fever is to start a broad-spectrum empiric antibiotic that would cover common organisms, such as cefepime, piperacillin-tazobactam, and meropenem. Gram positive coverage such as vancomycin should be added in hemodynamically unstable and suspected hospital-acquired pneumonia, catheter infection, and severe mucositis.5 Antibiotics are generally narrowed down based on the source of infection and culture results. If the fever persists for more than 3 days, there should be concern for a possible fungal infection, which would warrant fungal serology, imaging, initial antifungal coverage, and a consultation to infectious disease.5 Duration of antibiotics is typically decided based on the source, organism, and nature of infection. At a minimum, antibiotic should be continued until the patient’s ANC is >500/µL.5 In many patients with hematologic cancers, such as myelodysplastic syndrome or acute leukemia, adequate count recovery may not happen and decision is based on clinical ground.
Tumor Lysis Syndrome
As name implies, tumor lysis syndrome (TLS) occurs after acute lysis of tumor cells spontaneously or following chemotherapy. The types of cancers that typically causes TLS are often ones with a high cell turnover, such as acute leukemia and high-grade lymphomas or cancers that are highly responsive to chemotherapy.6 Of the hematologic cancers, acute leukemias and high-grade lymphomas have the highest rates of TLS. Of the solid tumors, patients with small cell lung cancer are sensitive to platinum based chemotherapy and are at risk of TLS.7 In general, bulky tumors (>10 cm), wide-spread metastasis and patients with preexisting renal insufficiency are at higher risk of TLS.8
Due to the rapid release of intracellular contents, TLS results in profound electrolyte imbalance. Uric acid and phosphate crystals cause acute renal injury potentially leading to irreversible kidney damage and dialysis.4 Life-threatening cardiac arrythmia and tetany may happen due to hyperkalemia and hypocalcemia, respectively.6 The Cairo-Bishop Criteria details that for clinical diagnosis of TLS two laboratory criteria or one clinical criterion is needed. Management for TLS involves adequate hydration, allopurinol, rasburicase and correction of electrolyte imbalances.4 Allopurinol, a xanthine oxidase inhibitor, prevents formation of uric acid and thus reduces the uric acid load. When uric acid is high, rasburic acid, a recombinant urate oxidase enzyme, is used to convert insoluble uric acid to highly soluble allantoin which is readily excreted through the kidneys. Frequent cardiac and electrolyte monitoring is critical part of the management of TLS. Dialysis may be needed in refractory, life-threatening cases.4
Malignant spinal cord compression
Malignant spinal cord compression (MSCC) is defined as the event when the tumor compresses, displaces or encases the spinal cord or cauda equina due to metastatic spread or direct invasion. Breast, lung, and prostate cancers are among the most common solid tumors causing MSCC. Multiple myeloma and lymphoma are the most common among hematologic cancers.3 MSCC is commonly located in the thoracic spine, which accounts for 60% of cases, while lumbosacral accounts for 30% and cervical 10%.3
The most common presentation is back pain and typical red flag syndromes, such as motor or sensory deficits, urinary retention, and bowel incontinence, which may not be apparent in all cases.9 Once suspected, an MRI of the whole spine should be done emergently, which will dictate management. Delay in diagnosis and treatment may lead to irreversible complications such as motor weakness and paralysis.9 It is crucial to note that high-dose glucocorticoids should be started immediately which will eventually be tapered off when definitive management is undertaken.3 All patients should be assessed for surgical decompression. Patients with single level compression and controlled systemic disease are the best candidates for surgery.10 Surgical decompression should be followed up with radiotherapy in most cases to maximize survival and functional outcome. On the other hand, multilevel compressions with radiosensitive tumors, such as multiple myeloma, lymphoma, and small cell lung cancer, are better candidates for upfront radiation.10 From a quality-of-life perspective, every effort should be made to definitively treat patients with surgery if they are good surgical candidates and have a reasonable life expectancy.
Malignant Bowel Obstruction
Malignant bowel obstruction (MBO) is a complication that can be an initial presentation of some cancers such as colon cancer and ovarian cancer.11 MBO can also arise over the course of disease progression, as a consequence of prior bowel surgeries or poor management of opioid-induced complication. MBO is commonly seen in patients with gastrointestinal and colon cancers, ovarian cancer, peritoneal carcinomatosis or retroperitoneal metastases. Recurrence of MBO is common particularly in palliative settings. Complications from MBO depends on the location of the tumor. Complications can include luminal obstruction, impaired peristalsis, dysmotility, extramural obstruction, intussusception.11 Patients typically present with classic symptoms such as nausea, vomiting, abdominal pain, and constipation.
The initial diagnostic tool for MBO is to use a plain radiograph of the abdomen. Diagnosis is confirmed with a contrast CT scan of the abdomen.12 Definitive management is surgical intervention for acute obstructive intraluminal mass, particularly treatment naïve newly diagnosed patients.13 Surgery is not often feasible in patients with advanced cancer because of extensive intrabdominal or peritoneal metastases. Conservative medical management includes bowel rest, antiemetics, analgesic, hydration and correction of electrolyte imbalances.13 Using nasogastric tube should also be considered as a temporizing measure based on acuity of symptom and severity of obstruction. Antispasmodic and opioid analgesics are the mainstay of pain management. Octreotide can be used to reduce colonic secretion when standard conservative management is inadequate.11 Endoscopic gastric or colonic stenting may be useful for patients with extracolonic obstruction.13 The role of total parenteral nutrition is controversial and can only be justified in patients with low-cancer burden who are treated with a curative intent.11 For patients with advanced metastatic, refractory cancers or who have multilevel obstruction, venting gastrostomy is an effective palliative measure that can help to control nausea, vomiting, bloating and abdominal distension.13
Hypercalcemia of malignancy
Hypercalcemia of malignancy can affect up to 30% of cancer patients.14 An estimated 80% of the cases are due to humoral mechanisms, as a result of paraneoplastic secretion of parathyroid hormone-related protein (PTHrP), 1,25-dihydroxycholecalciferol, and rarely PTH.14 Paraneoplastic hypercalcemia is generally seen in squamous cell cancers (lung, head and neck), breast cancer, non-Hodgkin’s lymphoma.15 Osteolytic hypercalcemia contains nearly 20% of all hypercalcemia of malignancy cases and is seen in many cancers with skeletal spread such as breast cancer, multiple myeloma and prostate cancer.3 Extrarenal 1,25- dihydroxycholecalciferol is commonly seen in lymphomas.3
Regardless of the mechanism, hypercalcemia of malignancy has common symptoms that include nausea, abdominal pain, constipation, polyuria, bone pain, and confusion and is often a poor prognostic indicator.14 It is important to look for non-malignant causes of hypercalcemia and possible osseous metastases. Workup for hypercalcemia of malignancy should include serum measurements of ionized calcium, albumin, PTH,PTHrP, 25-hydroxyvitamin D, and calcitriol levels.3 Treatment involves administration of intravenous fluids with normal saline, calcitonin, bisphosphonates, and glucocorticoids.5 Loop diuretics can be considered after patient is adequately hydrated.14 Definitive management of the cancer is important to prevent recurrence.
Cancer-associated thrombosis (CAT)
Cancers are associated with a higher incidence of venous thromboembolism (VTE) with an incidence rate up to 5 times more than the general population and are the leading cause of non-cancer death in ambulatory cancer patients who are receiving systemic therapy.16 Pancreatic, ovarian, gastric and gynecologic cancers have a higher risk of VTE.
Direct-acting oral anticoagulants (DOAC) are the current standard for the treatment of CAT because of better or equal efficacy as well as decreased bleeding risk and better compliance than low-molecular weight heparin (LMWH) as shown in a number of retrospective and prospective studies.17 Among the DOACs, apixaban and rivaroxaban have better efficacy compared to LMWH.17 Edoxaban appears to increase bleeding risk while dabigatran has not been sufficiently studied in cancer patients.17
When a patient is diagnosed with an acute thrombosis, the initial step is to assess the need for acute intervention. For unstable patients, such as those with a massive pulmonary embolism with hemodynamic compromise, a catheter associated thrombectomy or thrombolysis may be indicated.17 For stable patients, DOACs can be started in ER. For patients considered for parenteral anticoagulation, LMWH is preferred over unfractionated heparin (UFH).18 UFH is preferred for patient with active risk of bleeding and creatinine clearance <30 ml/min.19 Anticoagulation should be continued for at least 6 months.19 Extended anticoagulation beyond 6 months is indicated for patients with active cancer, metastatic disease, or who are receiving chemotherapy.20 Inferior vena cava filters should only be used in patients with acute lower extremity deep vein thrombosis (DVT) for whom anticoagulation is contraindicated. Being a mechanical device, an IVC filter can prevent pulmonary embolism but can also increase the likelihood of lower extremity DVT.21 Studies have shown increased filter thrombosis, fracture and migration of filters, cardiac tamponade, and cardiac perforation.21 Retrievable filter should be used and should be retrieved as soon as feasible. If the filter cannot be retrieved, patients should remain on anticoagulation indefinitely.19
Immune Checkpoint Inhibitors and Immune-Related Adverse Effects
Immune checkpoint inhibitors (ICPi) are among the newest innovation in cancer treatment, which have shown great efficacy in the treatment of a variety of cancer types. Despite the benefits from these inhibitors, they are associated with immune-related adverse effects (irAE). Fortunately, most irAEs are of the low-grade category, as the incidence of high-grade irAEs are less than 20%.22 Common irAEs include thyroid dysfunction, skin rash, colitis, pneumonitis and hepatitis.22 Serious adverse effects such as myocarditis or encephalitis are rare.22 Treatment for immune-related adverse effects depends on the severity of the irAEs. Low-grade irAEs can be managed in the ambulatory settings by holding the drug with or without oral steroid. Patients with higher grade irAEs require hospital admission and treated with high dose steroid.23 Stronger immunosuppressive drugs can also be used if there is no response in 48-72 hours of starting the steroid.23 Steroids should be tapered slowly over 4-6 weeks.23 Upon resolution of symptoms, a rechallenge with the immunotherapy drug can safely done in patients with low-grade irAEs. Permanent discontinuation is recommended for patients with high-grade irAEs.23
Prevention of oncologic emergencies
Patient with cancer often present to the emergency department due to complications related to cancer progression or due to the adverse effects from the therapies they are receiving. It is important to be proactive and have a low threshold to diagnose and treat. Many oncologic emergencies are preventable with proactive measures taken in the ambulatory clinics. These may include use of colony stimulating growth factors with chemotherapy regimens which are associated with higher risk of neutropenic fever, early initiation of antibiotics in febrile neutropenia, adequate hydration and use of allopurinol to prevent tumor lysis syndrome, surgical interventions of impending cord compressions. Cancer pain and other symptoms should be managed and monitored in dedicated supportive care clinics. In addition, in many health systems, same-day acute care oncology clinics have been introduced to see patients with mild symptoms. All of these measures can reduce emergency department visits, decrease hospitalizations, and improve outcomes.
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. doi:10.3322/caac.21763
- Gould Rothberg BE, Quest TE, Yeung SJ, et al. Oncologic emergencies and urgencies: A comprehensive review. CA Cancer J Clin. 2022;72(6):570-593. doi:10.3322/caac.21727
- Lewis MA, Hendrickson AW, Moynihan TJ. Oncologic emergencies: Pathophysiology, presentation, diagnosis, and treatment [published correction appears in CA Cancer J Clin. 2011 Nov-Dec;61(6):420. Dosage error in article text]. CA Cancer J Clin. 2011;61(5):287-314. doi:10.3322/caac.20124
- Higdon ML, Atkinson CJ, Lawrence KV. Oncologic Emergencies: Recognition and Initial Management. Am Fam Physician. 2018;97(11):741-748.
- Halfdanarson TR, Hogan WJ, Madsen BE. Emergencies in Hematology and Oncology. Mayo Clin Proc. 2017;92(4):609-641. doi:10.1016/j.mayocp.2017.02.008
- Gupta A, Moore JA. Tumor Lysis Syndrome. JAMA Oncol. 2018;4(6):895. doi:10.1001/jamaoncol.2018.0613
- Lara PN Jr, Moon J, Redman MW, et al. Relevance of platinum-sensitivity status in relapsed/refractory extensive-stage small-cell lung cancer in the modern era: a patient-level analysis of southwest oncology group trials. J Thorac Oncol. 2015;10(1):110-115. doi:10.1097/JTO.0000000000000385
- Belay Y, Yirdaw K, Enawgaw B. Tumor Lysis Syndrome in Patients with Hematological Malignancies. J Oncol. 2017;2017:9684909. doi:10.1155/2017/9684909
- Patnaik S, Turner J, Inaparthy P, Kieffer WK. Metastatic spinal cord compression. Br J Hosp Med (Lond). 2020;81(4):1-10. doi:10.12968/hmed.2019.0399
- Spratt DE, Beeler WH, de Moraes FY, et al. An integrated multidisciplinary algorithm for the management of spinal metastases: an International Spine Oncology Consortium report. Lancet Oncol. 2017;18(12):e720-e730. doi:10.1016/S1470-2045(17)30612-5
- Soriano A, Davis MP. Malignant bowel obstruction: individualized treatment near the end of life. Cleve Clin J Med. 2011;78(3):197-206. doi:10.3949/ccjm.78a.10052
- Shariff F, Bogach J, Guidolin K, Nadler A. Malignant Bowel Obstruction Management Over Time: Are We Doing Anything New? A Current Narrative Review. Ann Surg Oncol. 2022;29(3):1995-2005. doi:10.1245/s10434-021-10922-1
- Franke AJ, Iqbal A, Starr JS, Nair RM, George TJ Jr. Management of Malignant Bowel Obstruction Associated With GI Cancers. J Oncol Pract. 2017;13(7):426-434. doi:10.1200/JOP.2017.022210
- Klemencic S, Perkins J. Diagnosis and Management of Oncologic Emergencies. West J Emerg Med. 2019;20(2):316-322. doi:10.5811/westjem.2018.12.37335
- Mirrakhimov AE. Hypercalcemia of Malignancy: An Update on Pathogenesis and Management. N Am J Med Sci. 2015;7(11):483-493. doi:10.4103/1947-2714.170600
- Fernandes CJ, Morinaga LTK, Alves JL Jr, et al. Cancer-associated thrombosis: the when, how and why. Eur Respir Rev. 2019;28(151):180119. Published 2019 Mar 27. doi:10.1183/16000617.0119-2018
- Farge D, Frere C, Connors JM, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20(10):e566-e581. doi:10.1016/S1470-2045(19)30336-5
- Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost. 2017;117(2):219-230. doi:10.1160/TH16-08-0615
- O'Connell C, Escalante CP, Goldhaber SZ, McBane R, Connors JM, Raskob GE. Treatment of Cancer-Associated Venous Thromboembolism with Low-Molecular-Weight Heparin or Direct Oral Anticoagulants: Patient Selection, Controversies, and Caveats. Oncologist. 2021;26(1):e8-e16. doi:10.1002/onco.13584
- Poudel SK, Reddy CA, Park DY, et al. Clinical Outcomes of Cancer-Associated Thrombosis Beyond 6 Months of Anticoagulation. Blood. 2019;134(Supplement_1):3458-3458. doi:10.1182/blood-2019-127581
- Nicholson W, Nicholson WJ, Tolerico P, et al. Prevalence of fracture and fragment embolization of Bard retrievable vena cava filters and clinical implications including cardiac perforation and tamponade. Arch Intern Med. 2010;170(20):1827-1831. doi:10.1001/archinternmed.2010.316
- Conroy M, Naidoo J. Immune-related adverse events and the balancing act of immunotherapy. Nat Commun. 2022;13(1):392. Published 2022 Jan 19. doi:10.1038/s41467-022-27960-2
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-1768. doi:10.1200/JCO.2017.77.6385